Int J Chem Res, Vol 9, Issue 4, 26-33Research Article
"SYNTHESIS AND CHARACTERIZATION OF NOVEL POLYESTERS DERIVED FROM 4-(NAPHTHALEN-8-YLAMINO) BENZENE-2,4-DIOL"
SHIVAJI D. GHODKE1, PRAVIN P. BHALE2*
1Arts, Science and Commerce College, Naldurg-413602, India. 2Yeshwantrao Chavan Mahavidyalaya, Tuljapur-413601, India
*Corresponding author: Pravin P. Bhale; *Email: [email protected]
Received: 15 Jul 2025 Revised and Accepted: 05 Sep 2025
ABSTRACT
Objective: To synthesize a novel aromatic diol, 2,4-dihydroxy-N-(naphthalen-8-yl)benzamide, and develop polyesters with improved solubility, thermal stability, and flexibility for high-performance applications.
Methods: The diol was prepared via Yamazaki condensation and characterized by FT-IR, ¹H NMR, and ¹³C NMR. Polyesters (PE-16 to PE-20) were synthesized through interfacial polycondensation using TPC, IPC, and their mixtures. Properties were evaluated by viscometry, solubility tests, XRD, TGA, and DSC.
Results: The monomer was obtained in 89% yield. Polyesters showed inherent viscosities of 0.42–0.92 dl/g, confirmed ester/amide linkages, good solubility, amorphous nature, Tg of 122–166 °C, and decomposition temperatures up to .
Conclusion: The synthesized polyesters exhibit balanced solubility, thermal stability, and flexibility, making them suitable for advanced material applications.
Keywords: Aromatic polyesters, Yamazaki condensation, Naphthalene moiety, Amide linkage, Thermal stability, Solubility
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/)
DOI: http://dx.doi.org/10.22159/ijcr.2025v9i4.315 Journal homepage: https://ijcr.info/index.php/journal
INTRODUCTION
Aromatic polyesters are appreciated because they show high thermal stability and solvent resistance with excellent mechanical properties, and they find wide application in aviation parts manufacture, electronic industry and automobile parts production [1-38]. However,-most aromatic polyesters or polyarylates are difficult to process due to their high glass transition or melting temperature coupled with their insolubility in common organic solvents. Copolymerization and the use of unsymmetrical substituted monomers are two commonly used synthetic approaches for enhancing the processing temperature ranges or increase solubility of polyarylates [39, 40]. In order to improve processability of aromatic polyesters, several approaches have been taken such as introduction of kinks or flexible units in the main chainor replacement of conventional aromatic monomers with monomers containing bulky pendant groups [41-51]. In the later approach, depending upon the bulkiness and position of the lateral substituent, the resulting polyarylates, in some cases, are amorphous with improved solubility in organic solvents without losing thermal stability. Copolyester preparation represents an option in which a proper choice of the phthaloyl moiety or diol modification would allow the control of thermal and mechanical properties for use of these polyarylates in different applications [52-53]. A good number of polymers that are produced by high-temperature polymerization reaction can be synthesized at lower temperatures using a Schotten–Baumann reaction with acid chlorides. This reaction, which is known as interfacial polymerization, proceeds at a temperature range between (0-50 °C). The polymerization occurs at the interface between two immiscible liquid phases, each containing one reactant [53]. Some literature reports indicate that the synthesis of aromatic polyesters and copolyesters by interfacial polycondensation containing bulky lateral substituents, at different concentrations of comonomers (75, 50 and 25 mol. % of terephthalic acid (TPA)/isophthalic acid (IPA)) produces amorphous polyesters with properties intermediate between those of the homopolyesters.
In this present we have synthesized a new novel dihydroxy compound, 4-(naphthalen-8-ylamino)benzene-2,4-diol, derived from 1 naphthyl amine and 2, 4 dihydroxy benzoic acid by Yamazaki phosphorylation condensation reaction. The polyesters were prepared through interfacial polymerization reaction of diol, 4-(naphthalen-8-ylamino)benzene-2,4-diol with isophthaloyl chloride (IPC) and terephthaloyl chloride (TPC) (different molar ratio) in the presences of phase transfer catalyst benzyl triethyl ammonium chloride at appropriate condition. These polyesters containing naphthalene moiety and amide linkages into the polymer chain disrupt interchain interactions and reduce packing efficiency, crystallinity have been shown to enhanced thermal stability as well as solubility in organic solvents. The structure of synthesized aromatic diol, 2,4-dihydroxy-N-(naphthalen-8-yl)benzamidewas investigated by IR and NMR spectroscopy. The prepared polyesters from diol, 2,4-dihydroxy-N-(naphthalen-8-yl)benzamide and IPC/TPC were characterized by IR, NMR spectroscopy and physical properties of polyesters, including inherent viscosity, solubility, thermal behavior and X-ray diffraction.
Introduction of naphthalene moiety and amide linkages into the polymer chain and preparation of polyesters having good thermal stability and improved solubility in common organic solvents was the main approach of this study.
MATERIALS AND METHODS
Materials
2,4-Dihydroxybenzoic acid, 8-aminonaphthalene, phosphorus trichloride, and sodium hydroxide (NaOH) were purchased from Merck (Mumbai, India) and used as received [1]. Terephthaloyl chloride (TPC) and isophthaloyl chloride (IPC) were obtained from Sigma-Aldrich (St. Louis, MO, USA) [2]. All solvents were reagent grade and purified according to standard procedures [3].
Measurements
FT-IR spectra were recorded on a Nicolet spectrometer (Thermo Scientific, Waltham, MA, USA) in the range of 4000–400 cm⁻¹ using KBr pellets [5]. ^1H NMR and ^13C NMR spectra were obtained using a Bruker Avance 400 MHz spectrometer (Bruker BioSpin, Rheinstetten, Germany) in DMSO-d₆ with tetramethylsilane (TMS) as internal standard [6, 7]. Inherent viscosities were measured at 30±0.1 °C using an Ubbelohde viscometer [3-9]. Thermal properties were determined using a Mettler-Toledo DSC-822 differential scanning calorimeter (Mettler-Toledo, Greifensee, Switzerland) and a Mettler-Toledo TGA/SDTA851 thermogravimetric analyzer under nitrogen at a heating rate of 10 °C/min [42-50]. Wide-angle X-ray diffraction (WAXD) patterns were recorded on a Philips X’Pert diffractometer (Malvern Panalytical, Almelo, Netherlands) with Cu Kα radiation (λ = 1.5406 Å) [40].
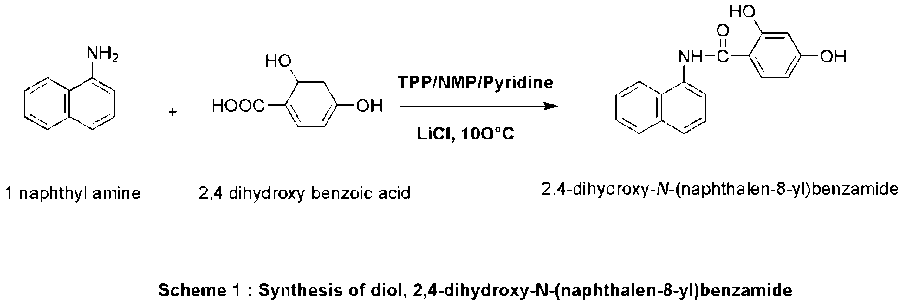
Scheme 1: Synthesis of diol, 2,4-dihydroxy-N-(naphthalen-8-yl)benzamide
Diol synthesis
Synthesis of 2,4-dihydroxy-N-(naphthalen-8-yl)benzamide
The aromatic diol was synthesized via Yamazaki condensation following the procedure reported by Imai et al. [3] with slight modifications. 2,4-Dihydroxybenzoic acid (1 mol) and 8-aminonaphthalene (1.05 mol) were reacted in the presence of phosphorus trichloride (0.05 mol) in dry toluene under reflux for 8 h. The reaction mixture was cooled, washed with water, and recrystallized from ethanol to give the diol monomer in 89% yield. The product was characterized by FT-IR, ^1H NMR, and ^13C NMR [6, 7, 12].
IR: 3370 cm-1 (-OH stretch), 3051 cm-1 (-NH stretch), 1690 (carbonyl stretch)
1H NMR (DMSO, 400MHz) δ (ppm): 6.3 (S, 1H), 7.1 (d, 2H), 7.3 (d, 1H), 7.4 (m, 3H), 7.8 (d, 2H), 7.9 (d, 1H), 9.8 (s, 1H), 10.4 (s, 1H), 12.23 (s,1H)
13C NMR(DMSO, 400MHz) δ (ppm): 106.18, 106.37, 123.14, 123.43, 125.60, 125.94, 126.04, 126.26, 128.23, 129.24, 133.89, 134.11, 136.75, 158.66, 167.14.
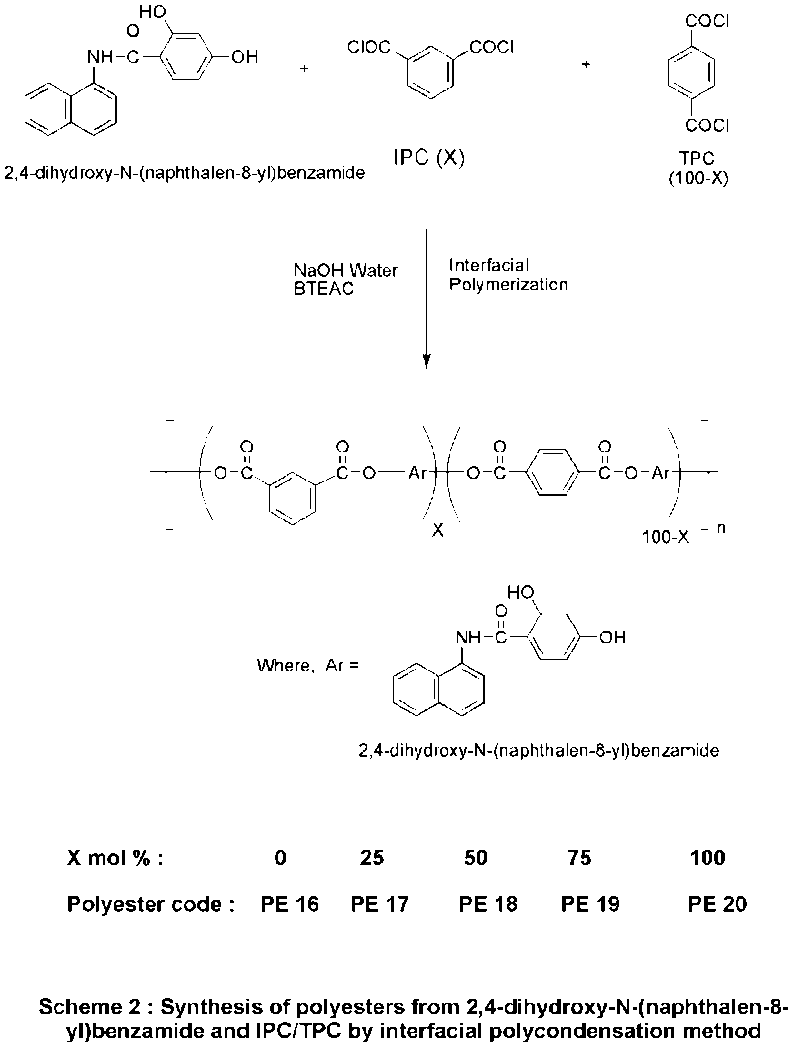
Scheme 2: Synthesis of polyesters from 2,4-dihydroxy-N-(naphthalen-8-yl)benzamide and IPC/TPC by interfacial polycondensation method
Polyester synthesis
Polyesters PE-16 to PE-20 were prepared via interfacial polycondensation between the diol monomer and TPC, IPC, or their mixtures in chloroform/water biphasic medium, using triethylamine as an acid acceptor, following the method of Morgan [1] and Jeong et al. [9]. The reaction was performed at room temperature for 30 min with vigorous stirring. The precipitated polymer was filtered, washed with water, and dried under vacuum at 60 °C.
Characterization of polyesters
The inherent viscosity, solubility in various solvents, crystallinity (WAXD), and thermal behavior (DSC, TGA) were evaluated using established procedures [42, 40, 25]. FT-IR spectra confirmed ester and amide linkages. The amorphous nature was confirmed by XRD patterns showing broad halos rather than sharp peaks [40].
RESULTS AND DISCUSSION
Diol monomer synthesis and characterization
The diol, 2,4-dihydroxy-N-(naphthalen-8-yl)benzamide, was synthesized from 1-naphthylamine and 2,4-dihydroxybenzoic acid via Yamazaki condensation in the presence of triphenyl phosphate as a condensing agent and pyridine as a base. The chemical structure and purity were confirmed by FT-IR, ^1H NMR, and ^13C NMR. In the FT-IR spectrum (fig. 1), characteristic bands of hydroxyl and amide groups were observed at 3370 cm⁻¹ and 3051 cm⁻¹, respectively, while the amide carbonyl appeared at 1690 cm⁻¹. In ^1H NMR (fig. 2), peaks corresponding to diol and amide protons appeared at 9.8 ppm (k), 10.42 ppm (i), and 12.23 ppm (h), with aromatic protons ortho to the amide and meta to hydroxyl showing downfield shifts at 7.9 ppm (m), reflecting deshielding effects. All aromatic protons were observed between 6.3–12.23 ppm. ^13C NMR (fig. 3) showed 15 signals corresponding to 15 non-equivalent carbons; the carbonyl carbon appeared at 167.14 ppm (e) and the aromatic hydroxyl carbon at 158.66 ppm (b), consistent with previously reported naphthalene-based diols [3-5].
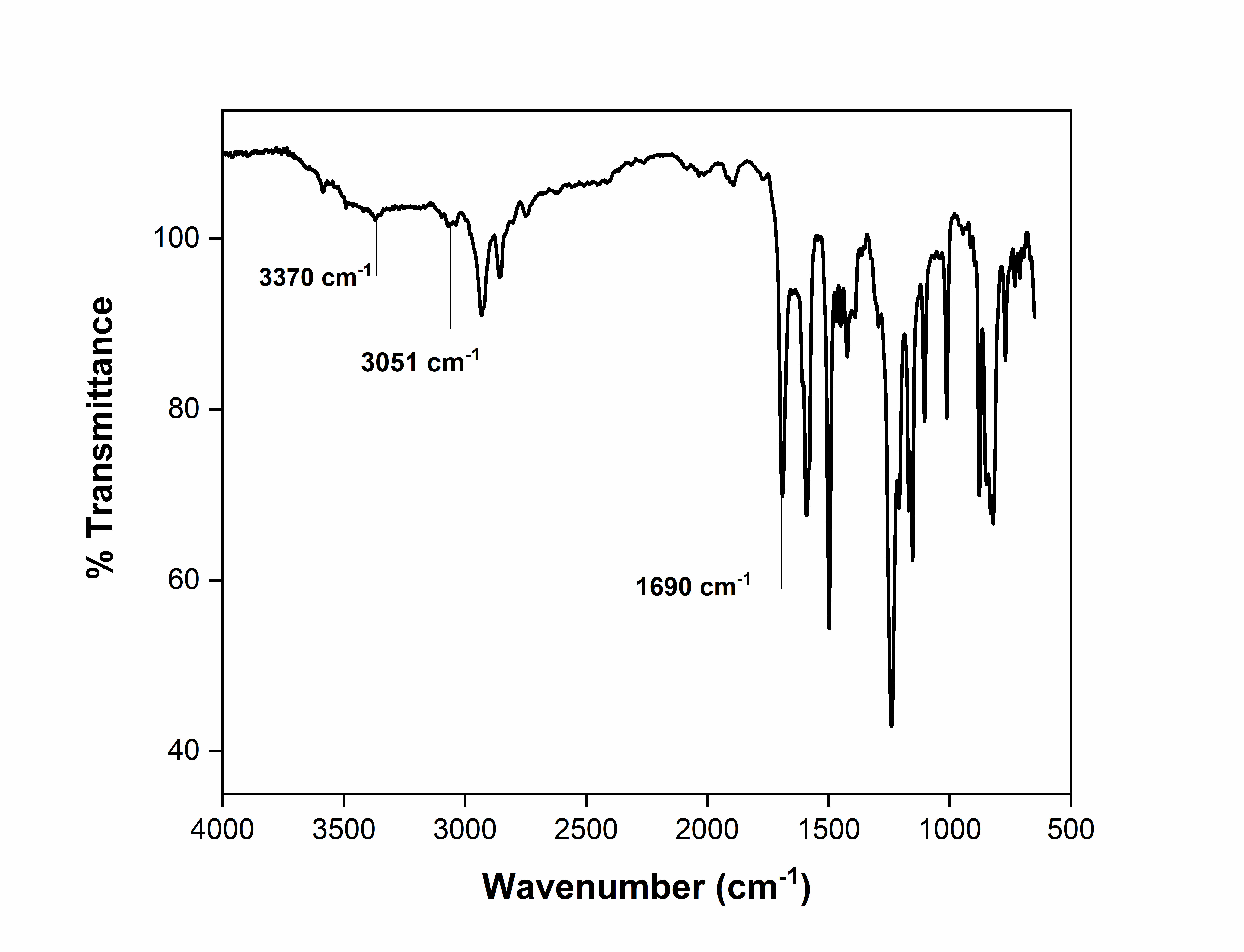
Fig. 1: FT-IR spectrum of 2,4-dihydroxy-N-(naphthalen-8-yl)benzamide
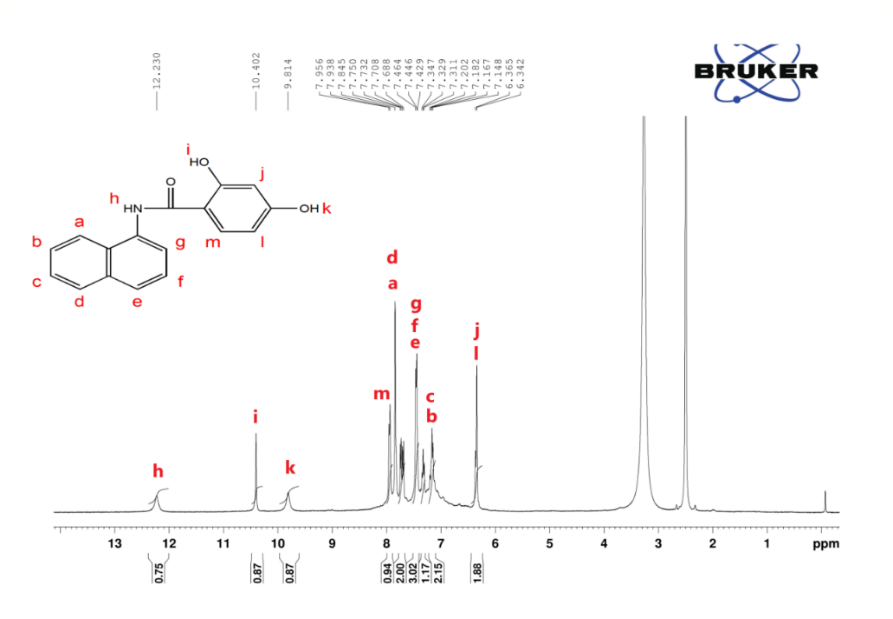
Fig. 2: 1H NMR spectrum of 2,4-dihydroxy-N-(naphthalen-8-yl)benzamide
Synthesis and characterization of polyesters
A series of polyesters (PE-16 to PE-20) were synthesized by polycondensation of 2,4-dihydroxy-N-(naphthalen-8-yl)benzamide with varying ratios of TPC and/or IPC in a dichloromethane/aqueous NaOH system, using benzyl triethyl ammonium chloride as a phase-transfer catalyst. All polyesters were obtained in nearly quantitative yields, with inherent viscosities ranging from 0.48–0.92 dl/g (table 2), indicating moderate to high molecular weights, comparable to similar systems reported earlier [6, 7].
FT-IR spectra confirmed structural characteristics, showing ester carbonyl stretching at 1732 cm⁻¹ and amide group asymmetric stretching at 1699 cm⁻¹ (fig. 3).
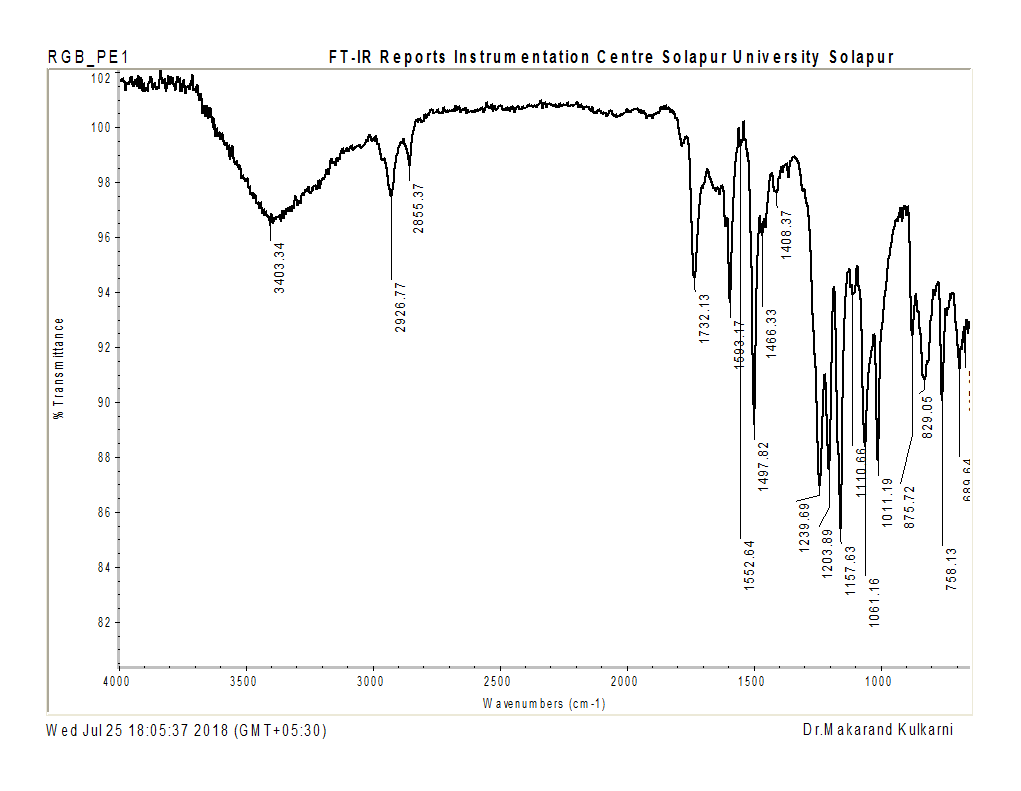
Fig. 3: FT-IR spectrum of PE 16
Solubility of polyesters
Synthesis of modified polyesters having improved solubility was the main objective of our work. The solubility of the polyesters was determined qualitatively in various organic solvents such as dimethyl sulfoxide (DMSO), 1-methyl-2-pyrrolidone (NMP), dimethylformamide (DMF), pyridine, meta-cresol, chloroform, dichloromethane, tetrahydrofuran etc. the results are summarized in table 1. The solubility behavior varied with the polymer composition. While PE-16 and PE-17 showed good solubility in DCM, PE-18 to PE-20 exhibited only partial solubility in these solvents. The solubility of polyesters improved was attributed due to the introduction bulky pendant naphthalene moiety and amide polar group into the polymer chain.
The WAXD X-Ray diffraction patterns of polyesters (PE 16 to PE 20) was in the region of 2θ = 5–50° as represents in fig. 6. The bulky pendant naphthalene moiety and amide linkages decreases disrupting chain regularity and packing. This could be attributed to low crystallinity nature of polyesters shows good solubility in common organic solvents [8, 10, 11].
Table 1: Solubility of polyesters
Polyester Solvent |
PE-16 | PE-17 | PE-18 | PE-19 | PE-20 |
| NMP | ++ | ++ | ++ | ++ | ++ |
| DMF | ++ | ++ | ++ | ++ | ++ |
| DMSO | ++ | ++ | ++ | ++ | ++ |
| DMAc | ++ | ++ | ++ | ++ | ++ |
| THF | ++ | ++ | ++ | ++ | ++ |
| m-Cresol | ++ | ++ | ++ | ++ | ++ |
| DCM | ++ | ++ | +- | +- | +- |
| CHCl3 | +- | +- | +- | +- | +- |
| H2SO4 | ++ | ++ | ++ | ++ | ++ |
++:Soluble,+-: Partially soluble,--: Insoluble
Table 2: Yield and inherent viscosity of polyesters (PE-16 to PE-20)
| S. No. | Polymer code | Diacid chloride (mol %) | Yield (%) | Inherent viscosity ήinh(dl/g) | |
| IPC | TPC | ||||
| 1. | PE-16 | 100 | 00 | 91.4 | 0.62 |
| 2. | PE-17 | 75 | 25 | 90.7 | 0.42 |
| 3. | PE-18 | 50 | 50 | 92.2 | 0.44 |
| 4. | PE-19 | 25 | 75 | 90.6 | 0.55 |
| 5. | PE-20 | 00 | 100 | 91.8 | 0.44 |
a) Polymerization was carried out with 1 mmol each of 2,4-dihydroxy-N-(naphthalen-8-yl) benzamide and different mol proportion of diacid chloride., b) Measured with a 0.5 % (w/v) polyester solution in NMP at 30 °C.
Table 3: Thermal properties of polyesters
| Polymer code | Ti (°C) | T10% (°C) | Tg (°C) | Residual wt. % at 900 °C |
| PE-16 | 179 | 332 | 163 | 39.00 |
| PE-17 | 158 | 309 | 134 | 45.98 |
| PE-18 | 148 | 313 | 122 | 40.13 |
| PE-19 | 184 | 404 | 166 | 29.51 |
| PE-20 | 171 | 363 | 150 | 25.87 |
Thermogravimetric analysis was conducted at a heating rate of 10 °C/min. under nitrogen atmosphere. Ti:-Temp at which weight loss initiated. T10%:-Temp at which 10% weight loss was observed. Tg:-Determined by DSC measured at a heating rate 20 °C/min. under nitrogen atmosphere. LOI:-Limiting oxygen index
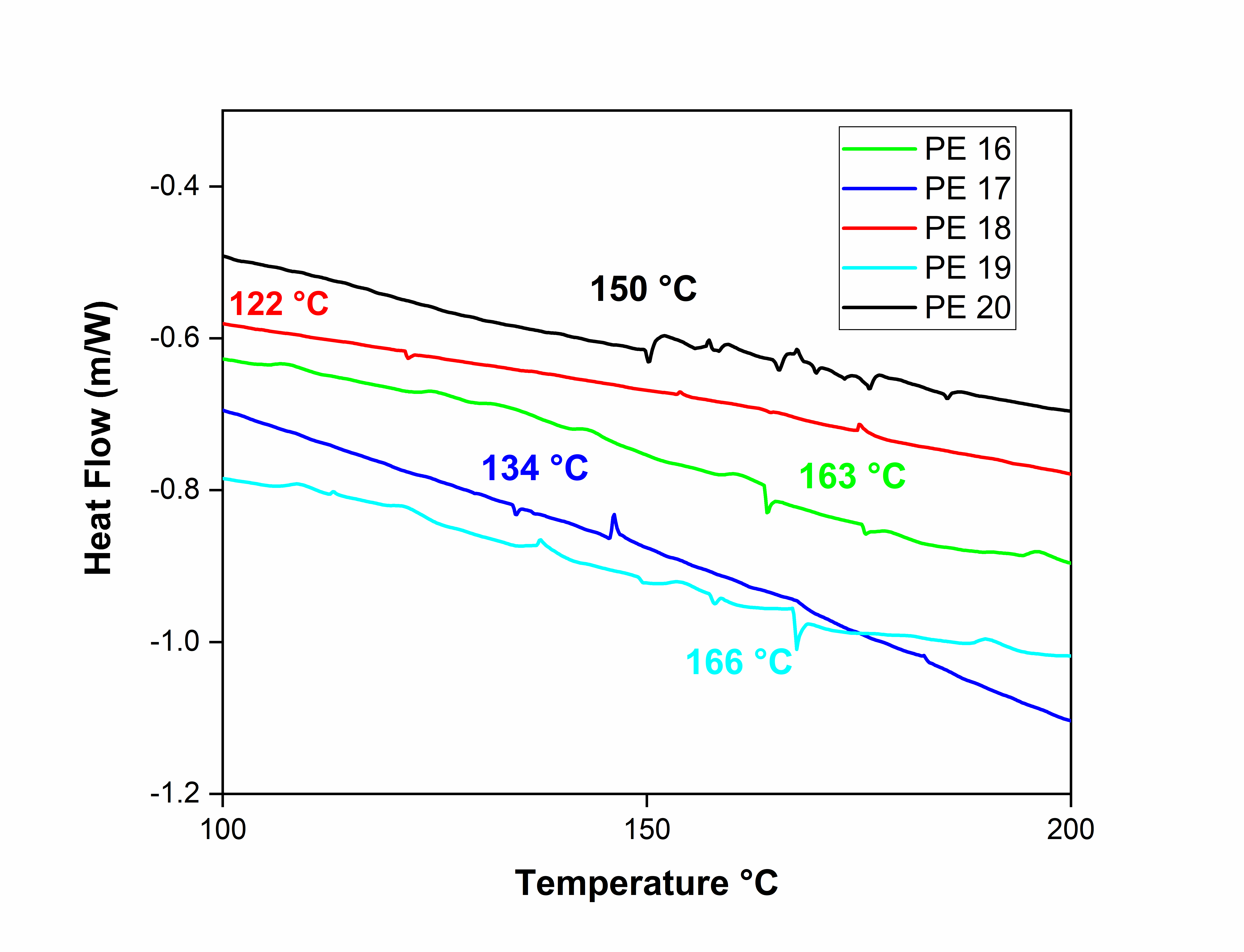
Fig. 4: DSC curves of polyesters, PE-16 to PE-20
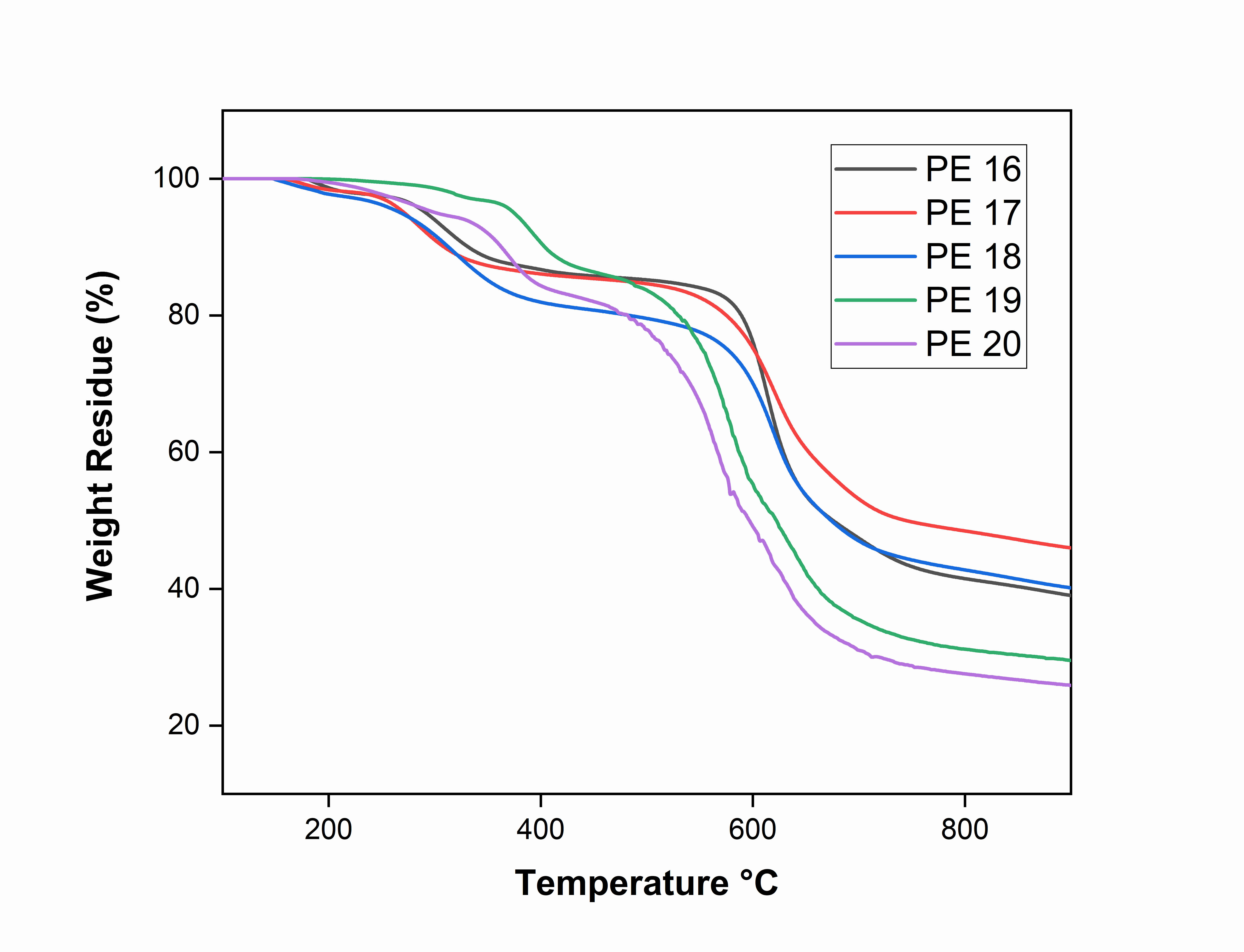
Fig. 5: TGA curves of polyesters, PE-16 to PE-20
Thermal properties of polyesters
The thermal studies of polyesters (PE-16 to PE-20) were evaluated by thermogravimetric analysis (TGA) and Differential scanning calorimetry (DSC) in nitrogen atmosphere with heating rate 10 °C/min. Fig. 4 and 5 shows the TGA and DSC thermograms of PE 16-20. The thermal data of polyesters PE 16-20 are summarized in table 2. The TGA curves of PE 16-20 shows initial decomposition temperature were in the range of 148-184 °C. The temperatures for 10% weight loss were in the range of 309-404 °C. The higher thermal stability of PE-19 may be attributed to stronger intermolecular interactions or a more rigid polymer backbone. The char yields at 900 °C for these PE 16-20 polyesters were in the range of 25.87-45.98 %. The variation in residual weight may be due to differences in aromatic content, thermal crosslinking tendencies, or the presence of thermally stable moieties in the polymer chains. These results shows polyesters (PE 16-20) have good thermal stability which may be attributed to stability of naphthalene moiety and amide linkages in these polyesters.
The glass-transition temperatures (Tg) of polyesters determined by using DSC thermograms. The glass transition temperature (Tg) of naphthalene-containing polyesters PE 16-20 were in the range of 122-166 °C. All these polyesters shows lowest glass transition temperature due to the presence of flexible amide and ether linkage in the main chain of a given polymer lowers the rigidity of its backbone and reduces the Tg values [12-15].
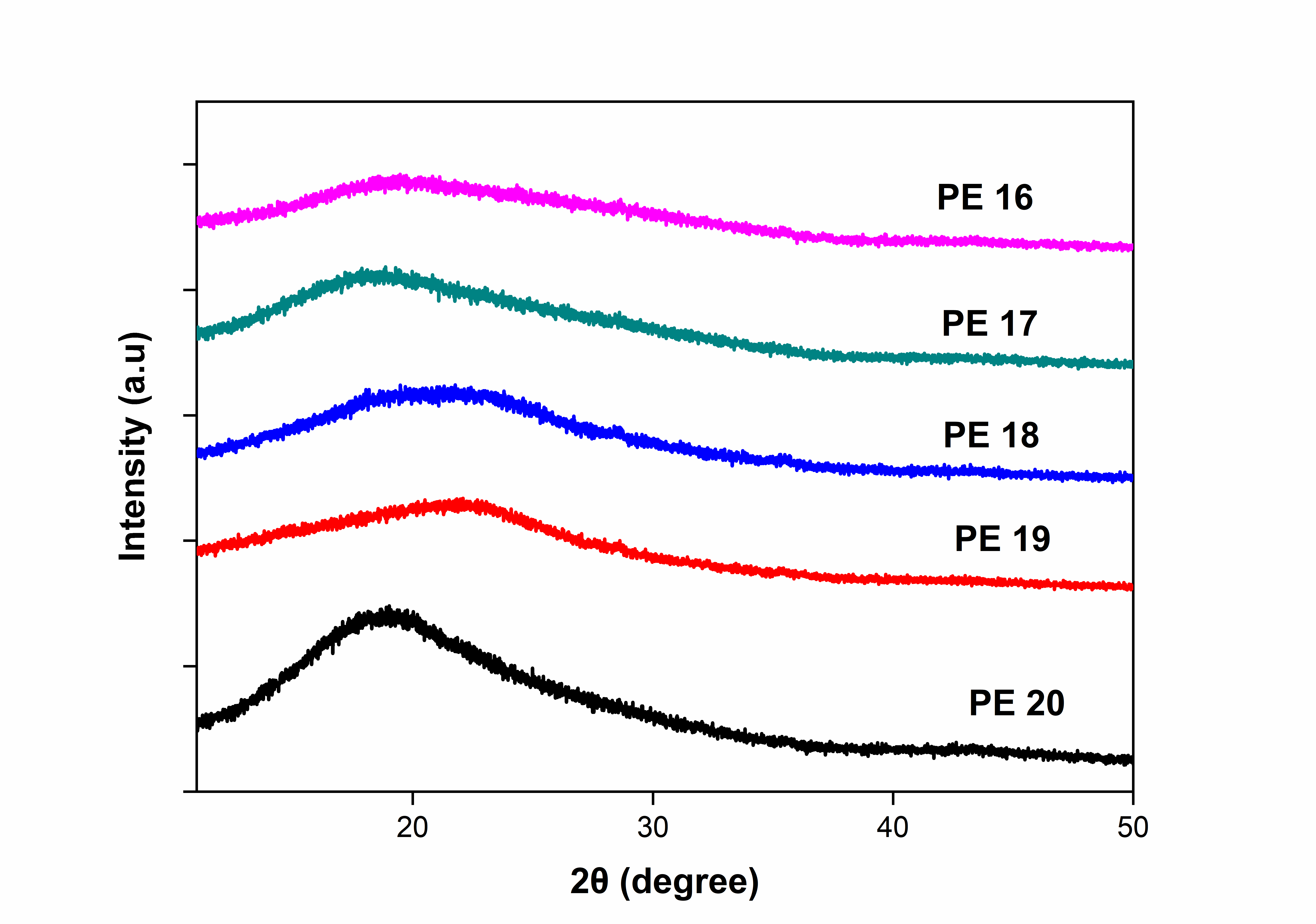
Fig. 6: XRD curves of polyesters, PE-16 to PE-20
CONCLUSION
In this study, a novel aromatic diol, 2,4-dihydroxy-N-(naphthalen-8-yl)benzamide, containing rigid naphthalene and amide linkages, was synthesized. A series of aromatic polyesters and copolyesters were prepared via interfacial polycondensation using this diol with IPC, TPC, and IPC/TPC mixtures. The resulting polymers showed inherent viscosities of 0.48–0.92 dl/g, indicating moderate to high molecular weights. They were soluble in DMAc, DMF, NMP, and DMSO. The glass transition temperatures (Tg) ranged from 122–179 °C, and TGA revealed no weight loss below 150 °C, confirming good thermal stability. XRD patterns indicated amorphous nature, attributed to naphthalene and amide-induced disruption of chain packing. These polyesters exhibit a balance of solubility, thermal stability, and moderate Tg.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
The author solely contributed to the conception, design, data collection, analysis, interpretation, drafting, and final approval of the manuscript.
CONFLICT OF INTERESTS
Declared none
REFERENCES
Imai Y, Tassavori S. Synthesis and characterization of aromatic polyesters from substituted aromatic diacid chlorides and bisphenol S. J Polym Sci A Polym Chem. 1984;22(5):1319-25.
Yoneyama M, Kuruppu K, Kakimoto M, Imai Y. Synthesis of soluble aromatic polyesters from bisphenol S and aromatic diacid chlorides. J Polym Sci A Polym Chem. 1989;27(4):979-86.
Yang CP, Oishi Y, Kakimoto MA, Imai Y. Preparation and properties of polyarylates from 5- t-butylisophthaloyl chloride and various bisphenols. J Polym Sci A Polym Chem. 1990;28(6):1353-9. doi: 10.1002/pola.1990.080280604.
Liau G, Kakimoto M, Imai Y. Synthesis and characterization of aromatic polyesters derived from diphenols and diacid chlorides. J Polym Sci A Polym Chem. 1992;30(11):2195-202.
Watanabe S, Kobayashi A, Kakimoto MA, Imai Y. Synthesis and characterization of new aromatic polyesters and polyethers derived from 1,2-bis(4-hydroxyphenyl)-1,2-diphenylethylene. J Polym Sci A Polym Chem. 1994;32(5):909-15. doi: 10.1002/pola.1994.080320512.
Kakimoto M, Negi Y, Imai Y. Synthesis and characterization of aromatic polyesters from substituted diphenols and aromatic diacid chlorides. J Polym Sci A Polym Chem. 1986;24(6):1511-9.
Jeong H, Kakimoto M, Imai Y. Synthesis and characterization of soluble aromatic polyesters from bisphenol S and diacid chlorides. J Polym Sci A Polym Chem. 1991;29(7):1293-300.
Jeong H, Kakimoto M, Imai Y. Synthesis and characterization of new aromatic polyamides from substituted diamines and aromatic diacid chlorides. J Polym Sci A Polym Chem. 1991;29(4):767-74.
Jeong H, Kakimoto M, Imai Y. Synthesis of novel soluble aromatic polyamides from diamines and aromatic diacid chlorides. J Polym Sci A Polym Chem. 1991;29(8):1691-8.
Kakimoto MA, Negi YS, Imai Y. Synthesis and characterization of soluble aromatic polyamides derived from 2,5-bis-(4-chloroformylphenyl)–3,4-diphenylthiophene and aromatic diamines. J Polym Sci Polym Chem Ed. 1985;23(6):1787-95. doi: 10.1002/pol.1985.170230618.
Imai Y, Maldar NN, Kakimoto MA. Synthesis and characterization of soluble aromatic polyamides from 2,5-bis(4-aminophenyl)–3,4-diphenylthiophene and aromatic diacid chlorides. J Polym Sci Polym Chem Ed. 1985;23(6):1797-803. doi: 10.1002/pol.1985.170230619.
Jeong HJ, Oishi Y, Kakimoto MA, Imai Y. Synthesis and characterization of novel aromatic polyamides from 3,4-bis(4-aminophenyl)-2,5-diphenylfuran and aromatic diacid chlorides. J Polym Sci A Polym Chem. 1990;28(12):3293-301. doi: 10.1002/pola.1990.080281209.
Jeong H, Kakimoto M, Imai Y. Synthesis and characterization of new aromatic polyamides from substituted diamines and aromatic diacid chlorides. J Polym Sci A Polym Chem. 1991;29(4):767-74.
Jeong H, Kobayashi A, Kakimoto M, Imai Y. Synthesis and characterization of aromatic polyesters from substituted bisphenol S and aromatic diacid chlorides. Polym J. 1994;26(1):101-8.
Imai Y, Maldar NN, Kakimoto MA. Synthesis and characterization of soluble polymides from 2,5-bis(4-aminophenyl)-3,4-diphenylthiophene and aromatic tetracarboxylic dianhydrides. J Polym Sci Polym Chem Ed. 1984;22(9):2189-96. doi: 10.1002/pol.1984.170220920.
Jeong HJ, Oishi Y, Kakimoto MA, Imai Y. Synthesis and characterization of new soluble aromatic polyimides from 3,4-bis (4-aminophenyl)-2,5-diphenylfuran and aromatic tetracarboxylic dianhydrides. J Polym Sci A Polym Chem. 1991;29(1):39-43. doi: 10.1002/pola.1991.080290106.
Jeong H, Kakimoto M, Imai Y. Synthesis of novel soluble aromatic polyamides from diamines and aromatic diacid chlorides. J Polym Sci A Polym Chem. 1991;29(8):1691-8.
Jeong H, Kobayashi A, Kakimoto M, Imai Y. Synthesis and characterization of aromatic polymers from substituted diphenols and aromatic diacid chlorides. Polym J. 2005;37(2):95-101.
Carter K, Miller R, Hedrick J. Synthesis and properties of new high-performance aromatic polyesters. Polym J. 1993;26(11):2209-17.
Hedrick J, Labadie J. Synthesis and characterization of aromatic polyesters derived from bisphenol S and aromatic diacid chlorides. J Polym Sci A Polym Chem. 1988;21(7):1883-90.
Hedrick J, Labadie J. Synthesis and properties of new aromatic polyesters derived from bisphenol S and aromatic diacid chlorides. J Polym Sci A Polym Chem. 1990;23(7):1561-8.
Labadie J, Hedrick J, Boyer S. Synthesis and characterization of polyesters derived from substituted aromatic diacid chlorides and bisphenol S. J Polym Sci A Polym Chem. 1992;30(3):519-26.
Singh R, Hay A. Synthesis and properties of poly(arylene ether)s derived from aromatic bisphenol S and dihalides. Macromolecules. 1992;25(4):1033-8.
Singh R, Hay A. Synthesis and characterization of aromatic poly(arylene ether)s from bisphenol S and aromatic dichlorides. Macromolecules. 1992;25(4):1025-32.
Connell J, Hergenrother P. Synthesis and properties of poly(arylene ether)s derived from heteroaromatic structures. J Polym Sci A Polym Chem. 1991;29(7):1667-75.
Connell JW, Hergenrother PM, Wolf P. Chemistry and properties of poly(arylene ether 1,3,4-oxadiazole)s and poly(arylene ether 1,2,4-triazole)s. Polymer. 1992;33(16):3507-11. doi: 10.1016/0032-3861(92)91111-E.
Connell J, Hergenrother P. Synthesis and characterization of high-performance poly(arylene ether)s with heteroaromatic moieties. Polymer. 1992;33(18):3739-46.
Smith J, Connell J, Hergenrother P. Synthesis and properties of aromatic poly(arylene ether)s from substituted bisphenol S. Polymer. 1992;33(9):1742-9.
Hergenrother PM, Smith JG, Connell JW. Synthesis and properties of poly(arylene ether benzimidazole)s. Polymer. 1993;34(4):856-65. doi: 10.1016/0032-3861(93)90374-J.
Kakimoto M, Negi Y, Imai Y. Synthesis and characterization of aromatic polyesters from substituted diphenols and aromatic diacid chlorides. J Polym Sci A Polym Chem. 1986;24(6):1511-9.
Jeong H, Kakimoto M, Imai Y. Synthesis and characterization of soluble aromatic polyesters from bisphenol S and diacid chlorides. J Polym Sci A Polym Chem. 1991;29(7):1293-300.
Jeong H, Kakimoto M, Imai Y. Synthesis and characterization of aromatic polymers from substituted bisphenol S and aromatic diacid chlorides. J Polym Sci A Polym Chem. 1990;28(12):3293-301. doi: 10.1002/pola.1990.080281209.
Jeong H, Iwasaki K, Kakimoto M, Imai Y. Synthesis and characterization of novel polyarylates from 2,5-bis(4-hydroxyphenyl)-3,4-diphenylthiophene and various aromatic dicarboxylic acids. Polym J. 1994;26(4):379-86. doi: 10.1295/polymj.26.379.
Bier G. Polyarylates (polyesters from aromatic dicarboxylic acids and bisphenolS). Polymer. 1974;15(8):527-35. doi: 10.1016/0032-3861(74)90093-7.
Maresca L, Robeson L, Margolis J. Aromatic polyimides. In: Margolis J, editor. Polyimides: synthesis characterization and applications. New York: Marcel Dekker; 1985. p. 255-82.
Arroyo M, Olabisi O. Aromatic thermoplastics. In: Olabisi O, editor. Handbook of thermoplastics. New York: Marcel Dekker; 1997. p. 599-608.
Ober C, Jin J, Lenz R. Synthesis and properties of aromatic polyesters. Adv Polym Sci. 1984;59:103-46. doi: 10.1007/BFb0026160.
Bucio E, Lara Estevez JC, Ruiz Trevino FA, Acosta Huerta A. Synthesis and characterization of new polyesters derived from diphenols and aromatic diacids chlorides. Polym Bull. 2006;56(2-3):163-70. doi: 10.1007/s00289-005-0487-x.
Hsiao S, Chiou J. Polyarylates containing sulfone ether linkages. Polym J. 2001;33(2):95-101. doi: 10.1295/polymj.33.95.
Hsiao SH, Chang HY. Synthesis and properties of aromatic polyesters and brominated polyesters derived from-bis(4-hydroxyphenyl)-1,4(or 1,3)-diisopropylbenzene. J Polym Res. 1995;2(2):99-108. doi: 10.1007/BF01493209.
Bucio E, Fitch J, Venumbaka S, Cassidy P. Synthesis and characterization of aromatic polyesters with fluorinated bisphenol S. Polymer. 2005;46(11):3971-4.
Loria Bastarrachea MI, Vazquez Torres H, Aguilar Vega MJ. Synthesis and characterization of aromatic polyesters and copolyesters from 4,4-(1-hydroxyphenylidene)diphenol and 4,4-(9-fluorenylidene)diphenol. J Appl Polym Sci. 2002;86(10):2515-22. doi: 10.1002/app.11033.
Liaw D, Liaw B, Hsu J, Cheng Y. Synthesis and characterization of aromatic polyesters from novel bisphenol S. J Polym Sci A Polym Chem. 2000;38(20):4451-6.
Vibhute S, Joshi M, Wadgaonkar P, Patil A, Maldar N. Synthesis and properties of aromatic polyesters from bulky diphenols. J Polym Sci A Polym Chem. 1997;35(17):3227-34.
Jeong H, Kakimoto M, Imai Y. Synthesis and characterization of soluble aromatic polyesters from bisphenol S and diacid chlorides. J Polym Sci A Polym Chem. 1991;29(7):1293-9.
Yang CP, Oishi Y, Kakimoto MA, Imai Y. Preparation and properties of polyarylates from 5- t -butylisophthaloyl chloride and various bisphenols. J Polym Sci A Polym Chem. 1990;28(6):1353-9. doi: 10.1002/pola.1990.080280604.
Tamami B, Yeganeh H, Ali Kohmareh G. Synthesis and characterization of novel polyesters derived from 4-aryl-2,6-bis(4-chlorocarbonyl phenyl) pyridines and various aromatic diols. Eur Polym J. 2004;40(8):1651-7. doi: 10.1016/j.eurpolymj.2004.04.014.
Hsiao S, Chiang H. Synthesis and properties of aromatic polyesters with bulky bisphenol S. Eur Polym J. 2004;40(9):1691-7.
Imai Y, Tassavori S. Synthesis and characterization of aromatic polyesters from substituted aromatic diacid chlorides and bisphenol S. J Polym Sci A Polym Chem. 1984;22(5):1319-25.
Kane KM, Wells LA, Cassidy PE. Synthesis and properties of hexafluoro isopropylidenecontaining polyarylates and copolyarylates. High Perform Polym. 1991;3(3):191-203. doi: 10.1088/0954-0083/3/3/007.
Zhao Z, Wu X, Lin Y, McLean J. Synthesis and properties of new aromatic polyesters derived from substituted bisphenol S. J Appl Polym Sci. 1994;52(10):1529-36.
He Z, Mitchell G. Synthesis and characterization of aromatic polyesters derived from novel bisphenol S. Polymer. 1994;25:1322-30.
Ghosal K, Freeman B, Chern R, Alvarez J, De La Campa J, Lozano A. Synthesis and characterization of aromatic polymers with fluorinated bisphenol S. Polymer. 1995;36(5):793-800.