
Int J Chem Res, Vol 9, Issue 4, 11-16Review Article
ADVANCES IN DRIED BLOOD SPOT SAMPLING AND BIOANALYTICAL METHOD VALIDATION UNDER ICH M10 GUIDELINES: A COMPREHENSIVE REVIEW
SUBHRANSHU PANDA1 , TUSHAR CHAVAN2*
, TUSHAR CHAVAN2* , RAVINDRA BHAVSAR3
, RAVINDRA BHAVSAR3
1,2School of Pharmaceutical Sciences, Jaipur National University, Jagatpura, Jaipur, Rajasthan-302017, India. 3Department of Clinical, Pharmadesk Solutions Pvt Ltd, Navi Mumbai, Maharashtra-400710, India
*Corresponding author: Tushar Chavan; *Email: [email protected]
Received: 07 Jul 2025 Revised and Accepted: 28 Aug 2025
ABSTRACT
Adaptation of Dried Blood Spot (DBS) as a sample collection technique has gathered significant attention in recent years, which is due to multiple advantages. As this involves a collection of human or animal blood drop and putting them on the specialist paper, this provides multiple benefits like minimal invasive blood sample collection, room temperature or ambient temperature storage till analysis is over. However, all these advantages of DBS do not come easily. Before active application, scientific method development and then method validation as per regulatory requirements is very much required. To ensure achievement of DBS at an optimal level, it is very much essential that the bioanalytical method should be accurate and reproducible. Here International Council for Harmonisation (ICH) M10 guideline plays a pivotal role in supporting a framework for the method validation of methods that will used during regulatory submissions.
Keywords: Analytical chemistry techniques, Dried blood spot testing, Pharmacokinetics, Clinical trial
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/)
DOI: http://dx.doi.org/10.22159/ijcr.2025v9i4.312 Journal homepage: https://ijcr.info/index.php/journal
INTRODUCTION
A systematic search of the literature was carried out in PubMed, Scopus, Web of Science and Google Scholar. Keywords used included ‘dried blood spot’, ‘bioanalytical method’ and ‘bioanalytical method validation’, combined with Boolean operators (and, or). The search was limited to studies published between 1995 and 2025. Only peer-reviewed English-language articles were considered. Abstracts, theses, non-peer-reviewed reports and articles not related to human plasma analysis were excluded. References cited in the retrieved articles were also screened manually to identify additional relevant studies. Nowadays, research in pharmaceutical industry became more and more advance, which include requirement for human studies with requirement for detection at very low levels with consistent and accurate measurement of drug, biologics and biosimilar products in matrix sample. However, during this development, one cannot ignore the trial participants health and issues which arise because of clinical studies. Considering all these new DBS techniques is introduced, which is a kind of a very fruitful development, which helps to maintain human subject safety along with detection of analyte at very low level [1].
There is data available about DBS technique over the public domain and this data is continuously upgrading. However, our attempt includes research about DBS technique along with aspects like method development and method validation in line with well-known ICH M10 guideline. Developing a DBS method along with validation will provide a glimpse for use of DBS method in clinical studies [2].
So, in a nutshell, its simplicity, cost-effective, easiness and adaptability make it as a very good choice for pharmaceutical product development research [3].
Current scenario and applications
Traditional blood sampling and its limitations
The most common blood sampling collection method is venipuncture, which is widely used for decades. This technique is gold standard method and it receives acceptance globally and has its own advantages. However, this method doesn’t come with all advantages, it certainly has a limitation which forces researchers to look for alternative techniques like DBS sampling. The venipuncture method involves limitations like invasive procedures, requirement for skilled personnel, risk of infection, requirement of large blood sample volume and it is very much impractical for certain applications like pediatrics or geriatric population studies. Other alternative blood collection methods like, finger stick or capillary blood sampling, involve limitations like limited sample volume collection, blood composition may be different from actual blood, which impacting the accuracy of certain test and discomfort with pain. A similar type of issue is there with arterial blood gas sampling.
DBS technology
The limitations of traditional blood sampling methods have encouraged development and adoption of alternative techniques like DBS, which offers several advantages, including minimally invasive sampling, ease of transport, storage and applicability to various clinical and research scenarios [4]. A drop of blood is applied to a specific filter paper as part of processing blood samples, which is further subject to extraction to remove target components for quantitative analysis [5]. The benefits of this procedure are minimal blood volume needed for measurement and lack of plasma or serum separation. As it is understood, once biological samples get dried then pathogens usually get inactive due to a lack of water, which make DBS as best considering it can store at room temperature during transport [6].
Historical development and adoption
Human or animal blood sample collection by applying drop on specific paper using DBS sampling is an innovative clinical methodology. Robert Guthrie proposed this method first in 1963, which shows a positive side that can remove the requirement for venous blood collection, which is difficult technically when dealing with newborns and young kids [7].
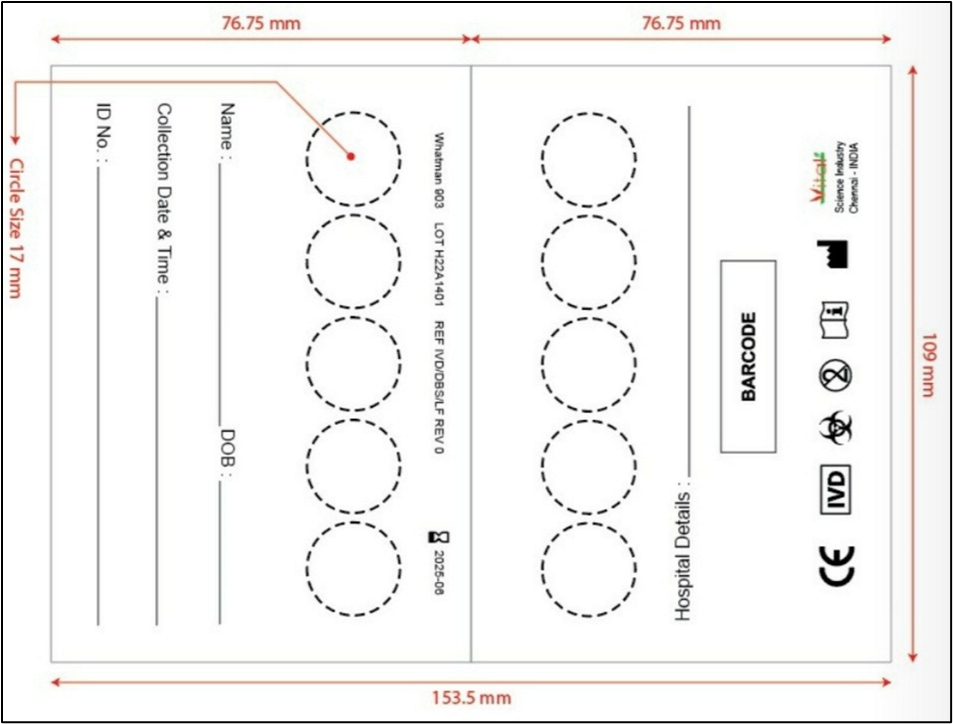
Fig. 1: Typical dried blood spot card for clinical study
Key applications of dbs in drug research
Drug development and pharmacokinetics (Pk)
In bioavailability studies, DBS method can be used to quantify drug products or analytes in biological Matrix such as blood or any other biological matrix across different time intervals to estimate bioavailability. In Pk profiling, DBS has an advantage that it can operate with low blood volume so it will help to get a detailed form of kinetic, which is particularly important. Similarly, in paediatric pharmacokinetics, DBS allows minimally invasive blood collection from infants and young children [8].
Clinical trials
In clinical studies. DBS is used to demonstrate the bioequivalence of generic and innovator drugs by comparing their Pk profiles. DBS enables longitudinal monitoring of drug concentrations, which further reduces need for frequent venipuncture. Similarly, in compliance-based monitoring, DBS can be used to monitor patient compliance by measuring drug levels in a convenient and minimally invasive manner [9].
Therapeutic drug monitoring (TDM)
Drug products which are having a very narrow therapeutic window require drug monitoring of patient. So, it helping to design drug dosage as per drug profile, which serve as personalized medicine. During home-based monitoring, patients can collect DBS samples at home, making TDM more accessible and convenient for chronic conditions like epilepsy or transplant patients. This data can guide clinicians for drug dosing adjustments in making timely dosing adjustments to optimize therapeutic outcomes with minimizing adverse effects [10].
Epidemiological and population studies
DBS is practical for large-scale epidemiological studies by allowing collection of blood samples from a geographically dispersed population. DBS can be used for disease surveillance and screening with resource-limited settings as it simplifies collection and transport [11].
Forensic and toxicology applications
Where sample volume is concern, DBS is used in forensic toxicology to measure drug and alcohol in criminal cases, accidents and post-mortem examinations. Also, DBS allows for easy and discreet monitoring of individuals in substance abuse rehabilitation programs [12].
Infectious disease monitoring
DBS is also widely used in HIV screening by allowing the collection and transportation of blood samples in areas with limited healthcare infrastructure [13]. DBS has been used in diagnosis and monitoring of various infectious diseases, including hepatitis, malaria and tuberculosis.
Key studies available over public domain
There are various studies which are published for public domain to suggest widespread adaptation and acceptance in various field, which includes clinical research, diagnostics and pharmacokinetics. Below are some important studies based on their innovative application in respective areas.
Guthrie and susi-1963
Robert Guthrie and Adele Susi are also considered pioneers of DBS, as they introduce a concept of using DBS for new-born screening of phenylketonuria. This ground-breaking work laid foundation for DBS in neonatal and paediatric healthcare.
Cunniff et al. 1998
Cunniff and their supportive researcher shows way with which it can be use in genetic screening. These researchers use DBS technique in condition like congenital adrenal hyperplasia during new-born screening [14].
Kissinger et al. 1981
Kissinger's demonstrated use of DBS in clinical Pk studies for drugs showcases the potential of DBS in monitoring drug levels [15].
Desfontaine et al. 2006
This research investigated application of DBS in neonatal screening for cystic fibrosis. It demonstrated efficacy of DBS in early diagnosis, contributing to improved management [16].
Marques et al.-2014
Marques and colleagues conducted a study on DBS for TDM of immunosuppressive drugs in transplant patients. Their findings underscored value of DBS in personalized medicine [17].
Guthrie and Pitt-1968
In this study, Guthrie and Pitt extended use of DBS to screen for sickle cell disease, demonstrating its versatility in detecting various genetic disorders [18].
Magera et al. 2006
This study focused on use of DBS for newborn screening of inborn errors of metabolism. It exemplified potential of DBS in high-throughput and multiplexed screening programs [19].
Owens et al. 2019
Owens and colleagues conducted research for use of DBS in monitoring drug levels in resource-limited settings, emphasizing its suitability for global health initiatives and decentralized healthcare [20].
Spooner et al. 2008
These researchers first time developed LC-MS/MS method for detection of acetaminophen using dog blood with just 15µl, which is a very low volume [21].
Abarca and Gerona 2023
Abarca evaluated applicability of DBS method for samples that were store for almost 17 y. These samples contained Alprazolam and its metabolite, which analysed using LC-MS/MS [22].
Steps during application of DBS
Use of DBS techniques in context of different studies mainly involved three steps; starting with method development, method validation and then application [23].
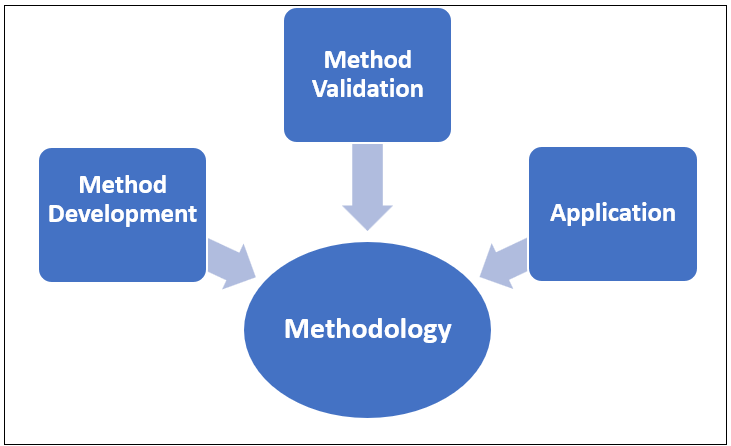
Fig. 2: Methodology of dried blood spot technique
Method development
The development of a DBS method is a systematic and iterative process that requires careful planning, validation with documentation to ensure its accuracy, reliability and applicability.
Procurement of DBS cards
A DBS card is a small piece of specialized paper used for collecting and storing blood samples. These cards are designed such that it allows convenient and minimally invasive collection of blood for various applications [24]. Currently, DBS cards that are in market for sampling are very standard and made of standardise cellulose or by synthetic standardise material. DBS card from Cytiva, which is sold in market as Whatman 903 is very widely used.
Under 21 Code of Federal Regulations 862.1675, the United States (USA) Food and Drug Administration has certified three DBS cards as medical devices for collection of blood specimens out of which we discussed above about Whatmann903. Apart from it, other two are Ahlstrom and PerkinElmer with code as 226-K062932 and 226-4 respectively [6]. Synthetic Bond Elut DMS Card from Agilent Technologies are also available for DMPK research. Guowen et al. demonstrated in their published research that a citric acid solution can be used on a card to increase medication stability through thiol-disulfide exchange [25]. Gabriel et al. created a disposable chip to address issue of uncertain blood volume and spots on traditional DBS cards with self-actuated dissolvable valves that measure exact volume of the blood.
Preparation of samples
The steps entail blood spotting on DBS card and then storing it at room temperature. Following extraction, clear liquid of analyte will be subjected to an analytical device for detection and measurement. Proteins, pathogens and enzymes are inhibited, preventing bacterial or viral growth. It is frequently used in pharmaceutical research and health care facilities, especially if blood sample volumes are difficult to collect and transport [26].
Chromatographic condition optimisation
Chromatographic conditions optimisations are crucial for accurate and reliable chemical separation and analysis. These conditions have a substantial impact on chromatographic method's efficiency, selectivity and resolution. The nature of analytes, chromatographic mode, stationary phase, detection method and regulatory requirements all influence chromatographic parameters selection for optimisation [27].
Optimization of extraction experiments
There are several punch tools available for DBS cards that are capable of cutting punches of varying sizes, which are then subjected to extraction so that they may be studied using analytical instruments without a considerable biological matrix that has potential to interfere with detection [28, 29].
Sample extraction can be performed using different techniques such as protein precipitation, liquid–liquid extraction, or solid phase extraction. Protein precipitation involves the addition of organic solvents to precipitate proteins, followed by collection of the supernatant. Liquid–liquid extraction is based on the addition of relatively non-polar organic solvents that allow the analyte to partition into the organic phase [30].
Solid phase extraction relies on a polar stationary phase to which the analyte adsorbs, thereby facilitating separation.
The application of an extraction method does not have to be the same every time, but it does require a science-based evaluation of molecule’s physical attributes and required level of detection, which is necessary. Most bioanalytical methods are quantitative rather than qualitative [24].
Method validation
Comprehensive advice on bioanalytical method validation is provided by the ICH M10 recommendation, which is especially important when it comes to validation of analytical techniques used to evaluate nonclinical and clinical pharmacokinetics of medications. International guidelines, such as ICH M10, should be followed during the validation process [31, 32].
Here is an overview of components typically included in a full validation as per ICH M10
Selectivity
In the Selectivity experiment, freshly collected blood from different donors gets spots on DBS paper to asses any interference, which is then analyse along with a freshly prepare calibration curve. It is first validation parameter to study in validation, where blank matrix, which is free from interference, will be considered for further validation activities.
Sensitivity
Sensitivity is usually assessed by analysing quality control samples at lowest concentration level on multiple occasions along with a freshly prepared calibration curve. This approach ensures that method’s ability to accurately and reliably detect lowest analyte concentration. Evaluating sensitivity as a validation parameter is critical, as it confirms method’s lower limit of quantification. This value is then used as a benchmark during actual sample analysis, ensuring that any measured concentrations falling below this validated sensitivity threshold are considered unreliable and thus excluded from final interpretation or reporting.
Specificity
The ability of a developed method to differentiate one analyte from other analytes in a matrix—which may be metabolites, isomers, or related to structure or drugs that may be administered to humans as part of a concurrent treatment—is known as specificity.
Matrix effect
The matrix effect is determined by analysing high-and low-quality control samples from several blood lots along with a fresh calibration curve sample. The passing or failing of these quality control samples indicates the extent to which matrix effect exists for developed method.
Precision and accuracy batch
During method validation, at least three Accuracy and Precision Batches should meet the batch acceptance criteria. This batch contains samples including a standard blank, a zero, which is simply a blank sample with an Internal Standard (IS), eight or more calibration curve standards and quality control samples at low, medium and high concentrations [33-35].
Carryover
Carryover is a methodological issue in which analytes from one sample are transferred to next sample from the instrument, which leads to false quantification. In a carryover experiment, a blank sample is typically injected adjacent to a high concentration sample following DBS processing in a sequential manner and amount of each analyte detected in the matrix blanks is then evaluated. If amount of analyte is limited or there is no interference found, then the method will be considered free from carryover.
Recovery
Recovery estimation involves evaluating efficiency of the method in extracting the analyte from biological matrix for accurate quantification. As per the ICH guidelines, recovery should be assessed at three concentration levels. Recovery is calculated by comparing analyte to IS area ratio obtained from extracted DBS samples with that from samples spiked directly into an organic solvent or blank extract.
Bench top stability in matrix
Bench Top stability evaluation entails subjecting spiked DBS samples to bench top environment conditions and then analysing them with freshly spiked DBS samples. This helps to determine how long continuous evaluation can be conducted in a laboratory environment.
Long-term stability in matrix
Long-term stability evaluation entails storing spiked DBS-spotted stability samples in a freezer for an extended period of time, which can range from the date of the study's first sample collection to the date of last sample analysis. After stability duration, the stability samples will be analysed to determine stability duration.
Application of method
Once DBS bioanalytical method meets acceptance for all validation parameters then same can be use in intended field of research. Method validation is documented evidence that shows confidence that method can precisely and consistently quantify analyte. Fig. 3 perfectly represent advantages of DBS method over conventional techniques if used during a clinical study.
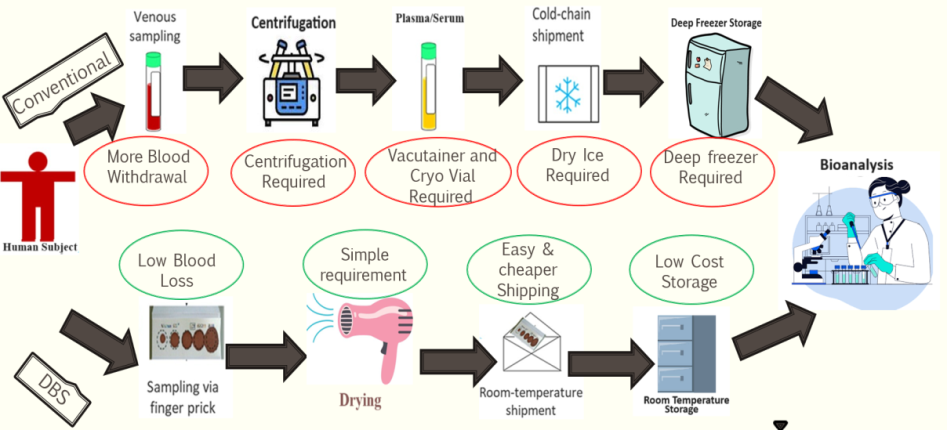
Fig. 3: Flow-wise advantage of dried blood spot technique over conventional biological matrix collection
CONCLUSION
The dried blood spot method, despite involving some complex processing steps, is considered green and environmentally friendly. It is especially suitable for pharmacokinetic and toxicokinetic studies due to its minimal blood volume requirement. DBS has potential to serve as a viable alternative to conventional plasma-based techniques in therapeutic drug monitoring and clinical research. With further validation, it may not only complement but potentially outperform well-established bioanalytical methods in terms of efficiency, sustainability and practicality.
A few rays of bright light are starting to set future of DBS sampling for regulatory drug bioanalysis. The discussion within scientific community is beginning to clarify way forward for dried matrix sampling. It has also been acknowledged that where dried matrix sampling has clear advantages over traditional wet sampling (for example, preclinical toxicology, paediatric studies, studies in remote locations), then constitute of the DBS data would be acceptable, provided that the data has been sufficiently validated according to the ICH M10 guideline.
ACKNOWLEDGEMENT
FUNDING
Nil
AUTHORS CONTRIBUTIONS
The present work is related to review of DBS and its method validation. All the authors contributed significantly to this manuscript, participated in reviewing/editing and approved the final draft for publication.
CONFLICT OF INTERESTS
Authors are not having any Conflicts of interests in subject matter included in this manuscript.
REFERENCES
Zijp TR, Izzah Z, Aberg C, Gan CT, Bakker SJ, Touw DJ. Clinical value of emerging bioanalytical methods for drug measurements: a scoping review of their applicability for medication adherence and therapeutic drug monitoring. Drugs. 2021;81(17):1983-2002. doi: 10.1007/s40265-021-01618-7, PMID 34724175.
Moein MM, Said R, Bassyouni F, Abdel Rehim M. Solid phase microextraction and related techniques for drugs in biological samples. J Anal Methods Chem. 2014 Feb 13;2014:921350. doi: 10.1155/2014/921350, PMID 24688797.
Stokes CS, Lammert F, Volmer DA. Analytical methods for quantification of vitamin D and implications for research and clinical practice. Anticancer Res. 2018;38(2):1137-44. doi: 10.21873/anticanres.12332, PMID 29374750.
Palnati N, Kotapati N, Vaidyanathan G. Liquid chromatography mass spectrometry/mass spectrometry method for the determination of lapatinib in rat plasma: application to pharmacokinetic studies in Wistar rats. Asian J Pharm Clin Res. 2021;14(2):74-7. doi: 10.22159/ajpcr.2021.v14i2.39660.
Permata D, Harahap Y, Ramadon D. Method development and validation of cefoperazone and sulbactam in dried blood spots by high performance liquid chromatography photodiode array detector. Int J App Pharm. 2022;14(5):214-9. doi: 10.22159/ijap.2022v14i5.45078.
Sharma A, Jaiswal S, Shukla M, Lal J. Dried blood spots: concepts present status and future perspectives in bioanalysis. Drug Test Anal. 2014;6(5):399-414. doi: 10.1002/dta.1646, PMID 24692095.
Michi M, Ferrari L. Validation of a high sensitivity assay for zatebradine in dried blood spots of human blood at pg/ml concentrations using HILIC-MS/MS. Bioanalysis. 2010;2(11):1863-71. doi: 10.4155/bio.10.126, PMID 21083494.
Stielow M, Witczynska A, Kubryn N, Fijalkowski L, Nowaczyk J, Nowaczyk A. The bioavailability of drugs the current state of knowledge. Molecules. 2023;28(24):8038. doi: 10.3390/molecules28248038, PMID 38138529.
Davit BM, Nwakama PE, Buehler GJ, Conner DP, Haidar SH, Patel DT. Comparing generic and innovator drugs: a review of 12 y of bioequivalence data from the United States Food and Drug Administration. Ann Pharmacother. 2009;43(10):1583-97. doi: 10.1345/aph.1M141, PMID 19776300.
Pandey A, Gupta SP. Personalized medicine: a comprehensive review. Orient J Chem. 2024;40(4):933-44. doi: 10.13005/ojc/400403.
Nain M, Sinha A, Sharma A. Dried blood spots: a robust tool for malaria surveillance in countries targeting elimination. J Vector Borne Dis. 2023;60(1):11-7. doi: 10.4103/0972-9062.373616, PMID 37026215.
Singh Z. Forensic toxicology: biological sampling and use of different analytical techniques. FRCIJ. 2017;4(4):111-20. doi: 10.15406/frcij.2017.04.00120.
Mavragani A, Ochoa G. Infoveillance of infectious diseases in USA: STDs, tuberculosis and hepatitis. J Big Data. 2018;5(1):1-23. doi: 10.1186/s40537-018-0140-9.
Dwyer JT, Cunniff PJ, Maroni BJ, Kopple JD, Burrowes JD, Powers SN. The hemodialysis pilot study: nutrition program and participant characteristics at baseline. The hemo study group. J Ren Nutr. 1998;8(1):11-20. doi: 10.1016/s1051-2276(98)90032-2, PMID 9724825.
Krum H, Bigger JT, Goldsmith RL, Packer M. Effect of long-term digoxin therapy on autonomic function in patients with chronic heart failure. J Am Coll Cardiol. 1995;25(2):289-94. doi: 10.1016/0735-1097(94)00417-O, PMID 7829779.
Desfontaine V, Guillarme D, Francotte E, Novakova L. Supercritical fluid chromatography in pharmaceutical analysis. J Pharm Biomed Anal. 2015;113:56-71. doi: 10.1016/j.jpba.2015.03.007, PMID 25818887.
Boso V, Herrero MJ, Bea S, Galiana M, Marrero P, Marques MR. Increased hospital stay and allograft dysfunction in renal transplant recipients with Cyp2c19 AA variant in SNP rs4244285. Drug Metab Dispos. 2013;41(2):480-7. doi: 10.1124/dmd.112.047977, PMID 23175667.
McClendon Weary B, Putnick DL, Robinson S, Yeung E. Little to give much to gain what can you do with a dried blood spot? Curr Environ Health Rep. 2020;7(3):211-21. doi: 10.1007/s40572-020-00289-y, PMID 32851603.
Magera MJ, Gunawardena ND, Hahn SH, Tortorelli S, Mitchell GA, Goodman SI. Quantitative determination of succinylacetone in dried blood spots for newborn screening of tyrosinemia type I. Mol Genet Metab. 2006;88(1):16-21. doi: 10.1016/j.ymgme.2005.12.005, PMID 16448836.
Owens CB, Szalanski AL. Filter paper for preservation storage and distribution of insect and pathogen DNA samples. J Med Entomol. 2005;42(4):709-11. doi: 10.1093/jmedent/42.4.709, PMID 16119565.
Barfield M, Spooner N, Lad R, Parry S, Fowles S. Application of dried blood spots combined with HPLC-MS/MS for the quantification of acetaminophen in toxicokinetic studies. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;870(1):32-7. doi: 10.1016/j.jchromb.2008.05.025, PMID 18550454.
Abarca R, Gerona R. Development and validation of an LC-MS/MS assay for the quantitative analysis of alprazolam α-hydroxyalprazolam and hydrocodone in dried blood spots. J Chromatogr B Analyt Technol Biomed Life Sci. 2023;1220:123639. doi: 10.1016/j.jchromb.2023.123639, PMID 36906954.
Tijare LK, Nt R, Un M. A review on bioanalytical method development and validation. Asian J Pharm Clin Res. 2016;9(9):6-10. doi: 10.22159/ajpcr.2016.v9s3.14321.
Ayre AP, Chaudhari PS, Shaikh J, Jagdale S, Agrawal O. Dried matrix spotting an innovative sample preparation tool in bioanalysis. Int J Pharm Sci Res. 2018;9(9):3597. doi: 10.13040/IJPSR.0975-8232.9(9).3597-07.
Liu G, Ji QC, Jemal M, Tymiak AA, Arnold ME. Approach to evaluating dried blood spot sample stability during drying process and discovery of a treated card to maintain analyte stability by rapid on card pH modification. Anal Chem. 2011;83(23):9033-8. doi: 10.1021/ac2023876, PMID 21995953.
He D, Wang Z, Yang L, Liu T, Yao Y, Mao Z. Modeling and optimization of the drug extraction production process. Scientific Programming. 2016;2016:1-15. doi: 10.1155/2016/3279423.
Keith W, John W. Principles and techniques of biochemistry and molecular Biology. 7th ed. Cambridge: Cambridge University Press; 2018.
Roskar R, Trdan T. Analytical methods for quantification of drug metabolites in biological samples. In: Calderon L, editor. Chromatography the most versatile method of chemical analysis. In Tech. 2012 Oct 24:440. doi: 10.5772/51676.
Bylda C, Thiele R, Kobold U, Volmer DA. Recent advances in sample preparation techniques to overcome difficulties encountered during quantitative analysis of small molecules from biofluids using LC-MS/MS. Analyst. 2014;139(10):2265-76. doi: 10.1039/c4an00094c, PMID 24633191.
Shankar EB, Naidu CG, Devaraju S, Rao KV, Ramachandra B, Rao YS. Chiral ionic liquid based vortex assisted enantio separation of s-(+) and r-(–) besifloxacin and evaluation of zeropoint energy by two phase liquid liquid extraction. Orient J Chem. 2024;40(1):194-201. doi: 10.13005/ojc/400124.
Sahoo CK, Sudhakar M, Sahoo NK, Ram S, Rao M, Panigrahy UP. Validation of analytical methods: a review. Int J Chromatogr. 2018;01:8. doi: 10.29011/IJCST-112.
Khanduri P, Gahtori A. Quantitative UV-spectrophotometric method for the analysis of teneligliptin HBR and metformin HCl in pharmaceutical dosage form: development and validation. Orient J Chem. 2025;40(6):1647-52. doi: 10.13005/ojc/400615.
Rahmania TA, Harahap Y, Sandy K. Azithromycin and oseltamivir quantification method developed and validated using liquid chromatography-tandem mass spectrometry in dried blood spot. Int J App Pharm. 2024;16(2):182-7. doi: 10.22159/ijap.2024v16i2.49051.
Raju GE, Pottendla S, Yaparthi S. Bioanalytical approach to ensitrelvir estimation using liquid chromatography tandem mass spectrometry and its application to pharmaceutical research. Asian J Pharm Clin Res. 2025;18(3):25-9. doi: 10.22159/ajpcr.2025v18i3.53760.