
Int J Chem Res, Vol 9, Issue 4, 1-10Review Article
LIGNOCELLULOSE BIOCONVERSION BY GUT MICROBIOTA OF SATURNIIDAE SPECIES: A SUSTAINABLE PATH TO BIOFUEL PRODUCTION
SHESHU MUNIVENKATAPPA*, MALLIAH SHIVASHANKAR
Department of Life Science, Bangalore University, Bangalore-560056, India
*Corresponding author: Sheshu Munivenkatappa; *Email: [email protected]
Received: 07 Jul 2025 Revised and Accepted: 28 Aug 2025
ABSTRACT
A promising and sustainable resource for the synthesis of biofuels is lignocellulosic biomass, which is mainly made up of cellulose, hemicellulose, and lignin. The potential of lignocellulose, a plentiful and renewable substrate, to produce second-generation biofuels that do not compete with food crops has attracted much attention. However, because lignocellulose is complex and resistant, its effective conversion into fermentable sugars remains a significant bottleneck. Biological approaches that mimic natural degradation processes are being investigated to solve this problem. One interesting example is the gut microbiota of insects in the Saturniidae family, like the eri silkworm (Samia cynthia ricini). These insects have co-evolved with specific gut bacteria that use enzymatic activity to break down plant polymers, allowing for adequate nutrient absorption from the lignocellulosic diet.
Cellulases, hemicellulases, and ligninases play a key role in the microbial breakdown of lignocellulosic biomass, which is examined in this review, along with its structural makeup and biochemical processes. We examine the enzymatic profiles and metabolic pathways of the gut microbiota in different Saturniidae species, as well as their composition, diversity, and function. The potential for using these naturally occurring microbial systems to produce industrial biofuel is highlighted. We also emphasise how metagenomics, enzyme screening, and synthetic biology can be combined to improve the efficiency of lignocellulose conversion. The biotechnological potential of Saturniidae gut microbes as a sustainable solution for lignocellulose bioconversion and advanced biofuel production is highlighted in this review by combining research from microbial ecology, insect physiology, and bioprocess engineering.
Keywords: Lignocellulosic biomass, Saturniidae gut microbiota, Samia cynthia ricini, Cellulolytic enzymes, Second-generation biofuels, Microbial bioconversion, Metagenomics, Synthetic biology
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/)
DOI: http://dx.doi.org/10.22159/ijcr.2025v9i4.306 Journal homepage: https://ijcr.info/index.php/journal
INTRODUCTION
Biofuels derived from lignocellulosic biomass are being studied more because of the rising demand for sustainable energy sources. Using non-food lignocellulosic materials like forestry waste and agricultural leftovers, second-generation biofuels address food security issues and advance environmental sustainability in contrast to first-generation biofuels, which depend on food crops [1]. This shift is more important now than ever because of the serious health and environmental issues associated with the usage of fossil fuels. The past century has seen a sharp rise in the usage of fossil fuels. Global emissions of carbon dioxide (CO₂) from industry and fossil fuels have more than sevenfold grown from 1940 to 2024, from an estimated 4.86 billion metric tonnes in 1940 to an estimated 37.41 billion metric tonnes in 2024 [2]. Around 75% of the world's CO₂ emissions come from burning fossil fuels. This growth significantly impacts climate change. The effects on health are equally worrisome. Over 5.1 million premature deaths worldwide are caused by the burning of fossil fuels each year, which accounted for 61% of the 8.3 million deaths in 2019 that were attributed to outdoor air pollution [3]. Outdoor air pollution causes about 2 million fatalities in India annually, with fossil fuels being a major contributor [4]. Furthermore, the financial burden is severe. Globally, air pollution from fossil fuels is predicted to cost $2.9 trillion per year, or around 3.3% of global GDP. Medical costs reduce productivity at work, and broader economic repercussions are some of these costs [5]. Given these obstacles, exploring sustainable alternatives is critical. However, due to its intrinsic complexity, lignocellulose requires effective breakdown mechanisms to realise its potential fully. The gut microbiota of insects that consume lignocellulose, especially those from the Saturniidae family, have attracted much attention lately because of their exceptional capacity to break down biomass. These microbial communities associated with insects possess a wide range of enzymes that can degrade complex plant polymers, providing a biological blueprint for the manufacture of sustainable biofuel [6-14].
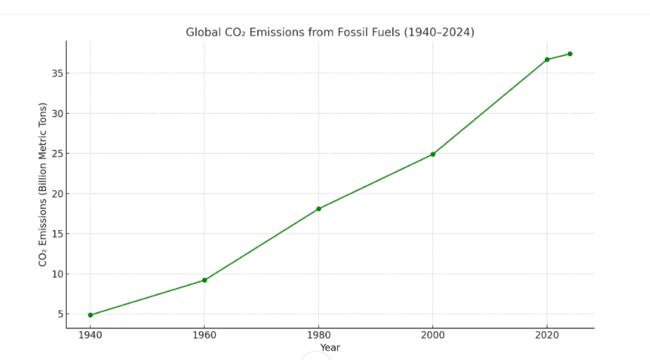
Fig. 1: Showing global CO₂ emissions from fossil fuels from 140 to 2024. The data illustrate the steady and significant increase in emissions over the decades, highlighting the environmental burden of fossil fuel reliance [2]

Fig. 2: Shows the global primary energy consumption by source (coal, oil, natural gas, and renewables) from 1950 to 2024. This graph depicts the continuously increasing utilisation of renewable energy sources vs the decades-long reliance on fossil fuels [3]
Biofuel generations and the promise of second-generation biofuels
Based on the source of their feedstock and the state of technology, biofuels may be roughly divided into four generations. This categorisation reflects biofuel research development, which addresses the trade-offs between sustainability, environmental effects, and food security [6, 7].
First-generation biofuels are made from edible biomass like vegetable oils, maize, and sugarcane. Even though these biofuels are widely utilised and technologically mature, their use raises concerns regarding food-fuel conflicts, changes in land use, and increased demand for agricultural inputs [8].
Second-generation biofuels use lignocellulosic biomass as feedstock, including agricultural wastes (e. g., maize stover and wheat straw), forestry waste, and non-food plants. These resources are more sustainable since they are abundant, inexpensive, and have less rivalry with food production. However, due to the resistant nature of lignocellulose, they require effective pretreatment and enzyme systems to be processed [9].
Third-generation biofuels are derived from microalgae and cyanobacteria, offering high lipid productivity and the potential for continuous cultivation in photobioreactors with relatively low land requirements [10].
Fourth-generation biofuels include sophisticated technologies that use genetically altered microbes or plants to boost photosynthetic efficiency and carbon absorption. These systems frequently combine synthetic biology and carbon-negative techniques to increase biofuel output [11].
Among all types, second-generation biofuels are seen as a practical and scalable solution due to the sheer volume of available lignocellulosic waste and their potential to reduce greenhouse gas emissions without jeopardising food security [12, 13].
Table 1: Illustrating comparative and summarising alternative methods for biofuel production
| Generation | Feedstock | Technology | Biofuel | Overall state | References |
| First-generation | Food crops (e. g., corn, sugarcane) | Fermentation, Transesterification | Ethanol, biodiesel | Commercial | [8] |
| Second-generation | Lignocellulosic biomass (e. g., crop residues, wood) | Pretreatment and enzymatic hydrolysis, Microbial fermentation, Gasification and catalysis | Ethanol, butanol, bio-based hydrocarbons | Developing | [9] |
| Third-generation | Algal biomass (e. g., microalgae) | Algae cultivation and extraction | Biodiesel, bio-based hydrocarbons | Developing | [10] |
| Fourth-generation | Modified feedstock or waste CO₂ | Metabolic engineering, Electrofuels | Advanced biofuels | Exploratory | [11] |
Saturniidae species adaptations
The Saturniidae family, including the eri silkworm (Samia cynthia ricini), has evolved remarkable adaptations in its digestive system, particularly through the presence of specialised gut microbiota that facilitates the degradation of plant-based polymers such as cellulose, hemicellulose, and lignin [14]. These microbes enable the insect to access energy from lignocellulosic materials, key structural components of plant cell walls, by producing enzymes that break down these otherwise recalcitrant compounds [15].
The gut microbiome of Samia cynthia ricini consists of cellulolytic, hemicellulolytic, and ligninolytic bacteria, each playing a distinct role. Cellulolytic bacteria such as Clostridium and Cellulomonas produce cellulases that hydrolyse β-1,4-glycosidic bonds in cellulose to release glucose [16]. Hemicellulolytic bacteria like Bacteroides and Prevotella produce xylanases and other enzymes to degrade hemicellulose into sugars such as xylose and arabinose [17]. Ligninolytic bacteria, notably those in the Actinobacteria phylum, provide enzymes such as lignin peroxidase and laccase, essential to break down lignin's complex aromatic structure [18]. These microbial enzymes have evolved to work in the insect stomach under low-oxygen and changing pH conditions, allowing Saturniidae species to consume high-fibre, woody plant components that other herbivorous insects commonly avoid [19].
Furthermore, the microbial population of Samia cynthia ricini stomach varies with developmental stage and season, showing a highly dynamic and sensitive system that responds to host demands and environmental inputs [14]. Saturniidae insects' mutualistic association with their gut flora promotes efficient digestion and helps to host immunity and physiological control [15].
Gut microbiota of saturniidae genera with lignocellulose-degrading capabilities
Gut microbiota of Actias species
The genus Actias, particularly Actias luna (Luna moth), is known for its larvae feeding on hardwood species like Betula, Juglans, and Liquidambar—plants that are rich in complex lignocellulosic fibres. To efficiently digest such fibrous diets, the larvae rely on a symbiotic relationship with gut microbiota capable of degrading plant structural polymers. Studies have demonstrated the presence of cellulolytic and hemicellulolytic bacterial genera such as Bacillus, Cellulomonas, Paenibacillus, and Pseudomonas in the midgut of Actias luna, many of which produce endo-glucanases and exo-glucanases, as well as β-glucosidases that synergistically hydrolyse cellulose into glucose [20-21].
In addition to cellulose, microbial xylanases and mannanases aid in the digestion of hemicellulose, which is mainly made up of xylans and mannans. These enzymes are required to disassemble the polysaccharide matrix that surrounds lignocellulosic fibres. Furthermore, metagenomic analysis suggests that the gut of Actias larvae harbours genes encoding carbohydrate-active enzymes (CAZymes), including glycoside hydrolases and carbohydrate esterases, indicating adaptation to host plants with varied polysaccharide profiles [20].
Specific microbial symbionts in Actias have been associated with roles beyond digestion. For instance, some Paenibacillus and Pseudomonas strains exhibit nitrogen-fixing capabilities, supporting protein synthesis in larvae that feed on low-nitrogen foliage. Others contribute to detoxification by degrading plant-derived phenolics and terpenoids, offering a survival advantage on chemically defended plants [22].
Gut microbiota of Antheraea species
Members of the genus Antheraea, including Antheraea pernyi (Chinese oak tussah) and Antheraea assamensis (muga silkworm), are key Saturniidae used in sericulture and are primarily folivorous on plants such as oak (Quercus spp.) and castor (Ricinus communis). These host plants contain lignocellulose and anti-nutritional and allelopathic compounds, necessitating a functionally versatile gut microbiome. The larval gut microbiota of Antheraea is dominated by lignocellulose-degrading genera such as Cellulomonas, Enterobacter, Bacillus, and Clostridium [21, 23]. These bacteria have high cellulolytic and xylanolytic capabilities, with numerous isolates capable of hydrolysing CMC, filter paper, and birchwood xylan in vitro. Clostridium species, in particular, are known for their anaerobic cellulolytic activity, which allows them to convert glucose into ethanol and organic acids, indicating their potential use in biofuel applications.
Antheraea's gut microbes also detoxify secondary compounds, including ricin and tannins. Enzymes like peroxidases, catalases, and laccases produced by gut microbes can neutralise reactive oxygen species and phenolic compounds in leaves, thereby reducing oxidative stress and improving larval health [22]. A wealth of genes linked to ABC transporters and efflux pumps is also found in microbial metagenomic investigations, which may be a factor in xenobiotic efflux and antibiotic resistance. The microbial makeup of Antheraea's gut varies with developmental stage, indicating a dynamic and flexible community structure that responds to changes in the host's food intake and physiological condition.
Gut microflora of Attacus species
The genus Attacus, particularly Attacus atlas (Atlas moth), represents one of the largest moth species globally and is characterised by a highly polyphagous larval stage. The larvae feed on diverse host plants such as Cinnamomum, Annona, Persea, and Mangifera, all abundant in complex lignocellulosic structures and plant defence chemicals. This polyphagy is underpinned by a robust gut microbiota that provides digestive and detoxification services. Isolates from the gut of Attacus atlas include functionally diverse bacteria such as Bacillus subtilis, Cellulomonas fimi, Pseudomonas fluorescens, and Enterobacter cloacae [21, 24]. These strains have been shown to produce high levels of extracellular cellulases, xylanases, and pectinases, facilitating the breakdown of major plant wall components. For example, Bacillus species exhibit endoglucanase activities exceeding 50 U/ml in optimised culture conditions, making them candidates for industrial applications. In addition to bacteria, fungi such as Candida tropicalis and Saccharomyces cerevisiae have been isolated from the larval gut, indicating a possible role in fermentative metabolism and converting sugars to ethanol. These fungal symbionts and bacteria may optimise the host's ability to produce energy from plant waste. Furthermore, Attacus larvae have a very alkaline midgut (pH>9), which not only boosts the activity of microbial enzymes but also aids in the solubilisation of lignin and cellulose, making them more susceptible to microbial assault. The gut's physiological environment may thus function as a bioreactor, optimising enzymatic hydrolysis at high pH levels [25].
Gut microbiota of Samia species
Samia cynthia ricini, the eri silkworm, has garnered increasing interest for its robust lignocellulose-degrading gut microbiota. Feeding predominantly on Ricinus communis, which is rich in lignocellulose and toxic compounds like ricin and ricinine, Samia cynthia ricini must rely heavily on gut symbionts for survival and digestion. Culture-dependent and molecular analyses have identified a suite of cellulolytic, xylanolytic, and ligninolytic bacteria in the gut, including Bacillus megaterium, Paenibacillus polymyxa, Microbacterium testaceum, Streptomyces spp., and Klebsiella spp. [22, 26]. These isolates exhibit hydrolytic activity on various substrates, including alkali lignin, birchwood xylan, filter paper, and CMC, suggesting a flexible enzyme system that breaks down plant biomass. Of particular interest is the detection of laccase and manganese peroxidase activity in certain Streptomyces and Bacillus strains, pointing to their potential role in partial lignin oxidation, an essential step in accessing embedded carbohydrates. This ability to initiate lignin depolymerisation sets Samia gut microbiota apart as a candidate model for biofuel-related bioconversion processes. Metagenomic investigations reveal the presence of carbohydrate-active enzymes (CAZymes) families in the gut glycoside hydrolase (GH) population, including GH5, GH10, GH11, and auxiliary activities 2 (AA2), which can disassemble lignocellulose. Furthermore, these microorganisms act as pathogen antagonists by secreting antimicrobial peptides and secondary metabolites that help maintain gut homeostasis and host defence. Notably, Samia gut symbionts have demonstrated tolerance to phenolic compounds and heavy metals in vitro, suggesting they could be employed in the bioremediation of lignocellulose-laden industrial waste streams and biofuel production [26].
Table 2: Explores the diversity of gut microbes from saturniidae silkworms and their enzymatic potential in biofuel production from lignocellulosic materials
| S. No. | Silkworm species | Biofuel potential | References |
| 1 | Samia cynthia ricini | Gut microbes produce cellulases and xylanases for efficient lignocellulose degradation. | [21, 27] |
| 2 | Antheraea pernyi | Hosts Clostridium and Cellulomonas, producing cellulolytic enzymes | [28] |
| 3 | Antheraea assamensis | Effective degradation of castor and oak leaves; potential for enzyme extraction | [29] |
| 4 | Attacus atlas | Actinobacteria and Clostridium produce lignin-degrading enzymes like laccase and peroxidase. | [27] |
| 5 | Actias luna | Contains cellulolytic and hemicellulolytic microbes, including Bacteroides, Clostridium | [16] |
| 6 | Citheronia regalis | Known to harbour Cellulomonas and other bacteria effective in cellulose breakdown | [30] |
| 7 | Eacles imperialis | Gut microbiota includes Bacteroides and Prevotella, producing hemicellulases. | [17] |
| 8 | Hyalophora cecropia | Bacterial symbionts degrade birch and cherry leaf biomass through enzymatic hydrolysis. | [19] |
Structure and composition of lignocellulose biomass
Lignocellulosic biomass is the most common renewable organic substance on Earth, consisting mainly of cellulose, hemicellulose, and lignin, which create a highly refractory matrix that acts as a structural framework in plant cell walls [31, 32]. This complex compound is resistant to microbial and enzymatic degradation, making successful breakdown critical in second-generation biofuel synthesis. Cellulose is the most prevalent polysaccharide in lignocellulose, accounting for 35–50% of its dry weight. The linear homopolymer of β-1,4-linked D-glucose units forms microfibrils with significant hydrogen bonding, resulting in a crystalline structure resistant to enzyme assault [33]. These microfibrils strengthen rods within the plant cell wall, considerably increasing tensile strength. Hemicellulose, a diverse collection of branching polysaccharides made up of pentoses (xylose, arabinose), hexoses (mannose, glucose, galactose), and sugar acids, accounts for 20-35% of lignocellulosic biomass [15]. Unlike cellulose, hemicellulose is amorphous and readily hydrolysed, but it forms a network with cellulose microfibrils and lignin, increasing the stiffness of the cell wall [34].
Lignin comprises 10–25% of the biomass, is a complex, non-polysaccharide aromatic polymer built from phenylpropanoid units—coniferyl, sinapyl, and p-coumaric alcohols [35]. Its hydrophobic nature and random three-dimensional structure confer compressive strength and protect carbohydrates from microbial degradation [36]. Lignin acts as a physical barrier, reducing the accessibility of hydrolytic enzymes to polysaccharides and thus presents a significant hurdle in lignocellulose bioconversion. The intimate association of cellulose, hemicellulose, and lignin within the plant cell wall forms a highly recalcitrant matrix to enzymatic hydrolysis. This structural complexity is further influenced by lignin–carbohydrate complexes (LCCs), covalent bonds that cross-link hemicellulose and lignin, impeding polysaccharides' separation and subsequent enzymatic processing [33, 35].
Plant species and growing circumstances alter these biopolymers' structural content and organisation, affecting the efficacy of biomass pretreatment and saccharification techniques. As a result, knowing the structural complexities of lignocellulosic biomass is critical for optimising biological and chemical conversion pathways for biofuel generation [37].
Insect gut microbiota as a natural source of lignocellulolytic enzymes
Insects feeding on fibrous plant materials have co-evolved with specialised gut microbiota, enabling efficient degradation of complex lignocellulosic substrates. These mutualistic interactions have made insects, particularly those from the Saturniidae family, a focus of bioprospecting for novel lignocellulolytic enzymes [38-39]. Species such as Samia cynthia ricini (Eri silkworm), Antheraea pernyi (Chinese oak tussah), and Attacus atlas (Atlas moth) harbour complex and dynamic gut microbial consortia. These microbes are adapted to the insect's plant-based diet and contribute to digestion by producing cellulases, hemicellulases, and lignin-modifying enzymes [27].
Recent breakthroughs in 16S rRNA gene sequencing and metagenomic profiling have revealed that the gut microbiota of Saturniidae insects is mainly formed of members of the Firmicutes, Proteobacteria, Actinobacteria, and Bacteroidetes phyla [21, 40-41]. These microbial combinations include Clostridium, Bacillus, Cellulomonas, and Pseudomonas, all of which have cellulolytic and xylanolytic capabilities [17]. Furthermore, many of these bacteria produce multifunctional enzymes, such as glycoside hydrolases and oxidative enzymes, which work together to break down the lignocellulosic matrix. This natural consortium model offers promising blueprints for designing microbial enzyme cocktails for industrial biomass conversion [16, 28]. The gut environment of these insects, characterised by a slightly acidic pH, short retention time, and nutrient-rich niche, also shapes the selection and activity of microbial species, making it a unique bioreactor for lignocellulose degradation studies [38].
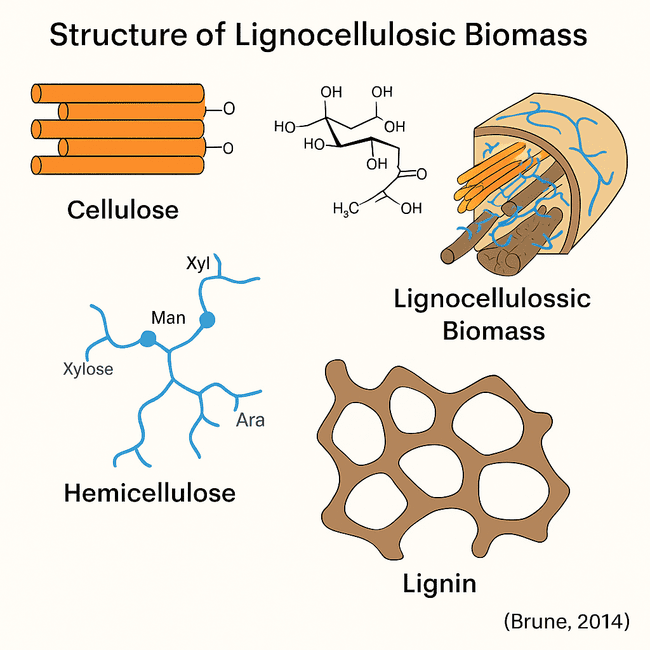
Fig. 3: This schematic illustrates the hierarchical organisation of lignocellulosic biomass in plant cell walls. The biomass is primarily composed of three polymers [38]
Mechanisms of lignocellulose degradation by gut microbes
Gut microorganisms' enzymatic breakdown of lignocellulosic biomass is a complex, multi-step process including a synergistic interaction of hydrolytic and oxidative enzymes. In lignocellulose-feeding insects such as those from the Saturniidae family, these enzymes are produced by symbiotic gut bacteria that have adapted to efficiently dismantle the complex plant polymers in the insect diet [16, 38].
Cellulases: deconstruction of cellulose
Cellulases are essential for the breakdown of cellulose, a crystalline homopolymer of β-1,4-linked glucose units. The microbial cellulolytic system often comprises Endoglucanases that randomly sever internal β-1,4 glycosidic linkages inside cellulose strands, producing oligosaccharides. Exoglucanases (cellobiohydrolases) release cellobiose units from the chain ends. β-Glucosidases hydrolyse cellobiose into glucose monomers, completing the saccharification process [42]. These enzymes often act synergistically, where endoglucanases increase substrate accessibility for exoglucanases, and β-glucosidases relieve product inhibition by hydrolysing accumulating cellobiose [43].
Hemicellulases: Targeting the heterogeneity of hemicellulose
Hemicellulose breakdown is more difficult due to its branching and heterogeneous structure, which includes sugars such as xylose, mannose, galactose, arabinose, and gluconic acid.
The key microbial enzymes are Xylanases, which degrade the backbone of xylan, the most prevalent hemicellulose polymer. Mannanases break down mannans and glucomannans. Accessory enzymes such as α-L-arabinofuranosidase, acetyl xylan esterase, and ferulic acid esterases remove side-chain decorations, boosting the activity of core hydrolases [44, 45]. Hemicellulases are often modular proteins with carbohydrate-binding modules (CBMs) that facilitate substrate targeting and processivity in complex plant matrices [46].
Ligninases: Overcoming the aromatic barrier
Lignin, the most refractory component of lignocellulose, creates a stiff network to protect cellulose and hemicellulose. Certain gut microorganisms, notably from insect hosts like termites and Saturniidae larvae, generate oxidative lignin-degrading enzymes, such as Laccases. Copper-containing oxidases catalyse the oxidation of phenolic lignin units, resulting in phenoxy radicals. Lignin and manganese peroxidases use hydrogen peroxide to oxidise non-phenolic lignin structures, breaking the aromatic rings [47, 48]. These enzymes are crucial not only for lignin depolymerisation but also for enhancing the accessibility of carbohydrate polymers, improving overall biomass digestibility [49].
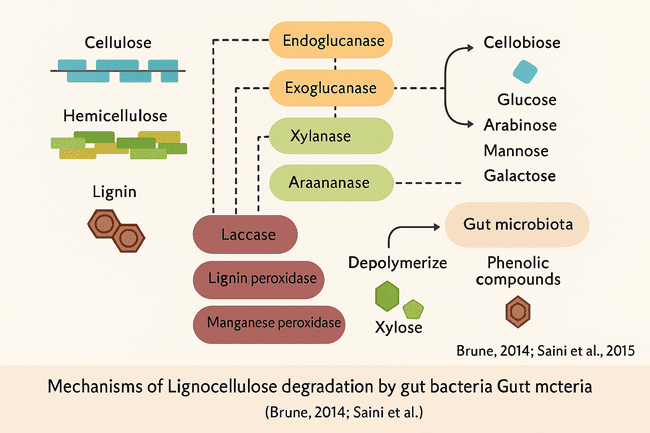
Fig. 5: Schematic representation of enzymatic pathways involved in cellulose, hemicellulose, and lignin degradation by gut microbiota [30, 48]
Metabolic pathways and bioenergy production
The conversion of lignocellulosic biomass into bioenergy consists of many coordinated processes, beginning with the enzymatic depolymerisation of complex plant polymers into fermentable monomers. Following this, the liberated sugars (such as glucose, xylose, arabinose, and mannose) and lignin-derived aromatic compounds are taken up by the microbial consortia in the insect gut and channelled through central metabolic pathways for energy production [50, 51]. This microbial conversion forms the core of second-generation biofuel production strategies.
Glycolysis and pentose phosphate pathway (PPP)
Monomeric sugars such as glucose and xylose are metabolised via glycolysis and the PPP, which are fundamental routes in both prokaryotic and eukaryotic cells. Glycolysis converts glucose to pyruvate, generating ATP and reducing equivalents like NADH, essential for downstream fermentation processes. Simultaneously, the PPP not only provides NADPH, crucial for biosynthetic reactions, but also contributes to the formation of precursors for nucleotides and aromatic amino acids and facilitates the metabolism of pentose sugars such as xylose and arabinose [50, 52].
Fermentation pathways
Microbial fermentation turns pyruvate into biofuels and value-added compounds under the anaerobic or microaerobic conditions found in insect guts. Common goods include pyruvate decarboxylation, which produces ethanol, which is then reduced by alcohol dehydrogenase. Butanol is produced in Clostridium species via the acetone-butanol-ethanol (ABE) fermentation cycle. Hydrogen gas, created by hydrogenase enzymes during fermentation, is a clean, transparent fuel with a high energy density. The end-products vary according to microbial species, cofactor availability, redox balance, and environmental circumstances [51, 53].
Aromatic compound degradation
Lignin decomposition produces complex aromatic compounds such as vanillin, ferulic acid, and guaiacol. Specialised gut bacteria metabolise these chemicals via funnelled routes that lead to central intermediates such as protocatechuate and catechol. These intermediates are subsequently ring cleaved by ortho or meta-cleavage pathways to produce acetyl-CoA, succinyl-CoA, or pyruvate, which enter the TCA cycle or fermentation routes. This not only improves energy harvesting, but it also lowers the toxicity of aromatic byproducts [18, 54].
Biofuel platform integration
Because of their metabolic flexibility, gut microbes are attractive candidates for metabolic engineering to increase the production of suitable biofuels. Synthetic biology enables the large-scale conversion of lignocellulose into high-value fuels by incorporating these processes into genetically tractable hosts (e. g., Escherichia coli, Saccharomyces cerevisiae) [55, 56].
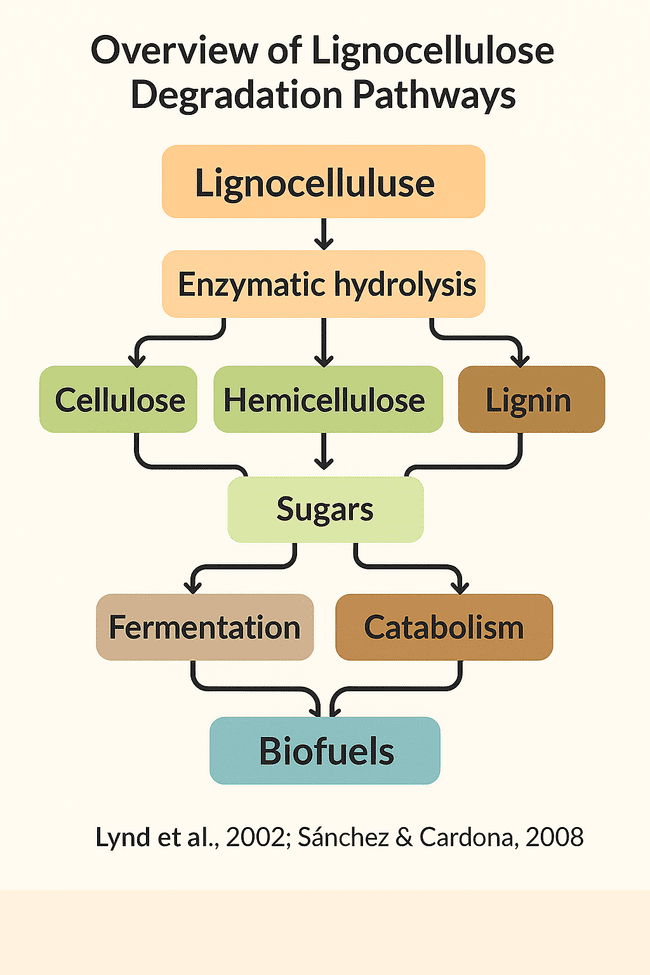
Fig. 6: Flowchart depicting the enzymatic breakdown of lignocellulose and subsequent metabolic pathways leading to biofuel production [42, 57]
Enzymatic profiling of gut microbiota
The enzymatic profiles of gut microbiota in Saturniidae insects must be investigated to discover microbial strains capable of digesting lignocellulose. These microorganisms are critical for breaking down the complex plant material in the insects' diets. By extracting bacterial strains from the gut and screening them for specific enzymatic activity, researchers can find microorganisms with the highest commercial potential, such as biofuel production [16, 58]. In vitro enzymatic assays are commonly used to assess the activity of lignocellulolytic enzymes, such as cellulases, xylanases, and ligninases. These assays use chromogenic or fluorogenic substrates to detect the presence and activity of enzymes in microbial extracts. For example, Congo red is used to detect cellulase activity, as it forms a red complex with cellulose degradation products, which can be visualised as colourimetric changes. Similarly, Remazol Brilliant Blue-Xylan is employed for xylanase activity, where the breakdown of xylan results in a decrease in blue colouration. For ligninases, substrates such as guaiacol or ABTS detect oxidation of lignin components, evidenced by a change in colour or the secretion of a zone roughly equivalent to bacterial colonies [59, 60].
High-performing isolates usually combine cellulolytic and hemicellulolytic activity, indicating that the enzymes work together to break down biomass efficiently. Endoglucanases and β-glucosidases break cellulose into glucose, while xylanases and β-xylosidases break down hemicellulose into sugars (e. g. xylose). These enzymatic systems enable microbial populations in the insect gut to digest a wide range of lignocellulosic substrates, boosting nutrient intake and energy production. These enzyme activity profiles indicate the gut microbiome's functional variety and serve to identify prospective bacterial candidates for downstream applications such as enzyme synthesis, recombinant gene expression, and centralised bioprocessing in biofuel industries [61, 28].
Gut microbiota dynamics in lignocellulose degradation
Saturniidae insects, like Samia cynthia ricini (eri silkworm), rely substantially on gut bacteria to degrade lignocellulosic biomass. These insects support a diverse microbial community that includes bacteria, fungi, and archaea, all of which help decompose cellulose, hemicellulose, and lignin. The microbial community in these insects' guts is highly specialised, allowing for the fast breakdown of lignocellulosic materials, often resistant to enzymatic hydrolysis in many species due to their complex and refractory structure [62, 63].
Key bacterial genera found in these insects' guts include Cellulomonas, Clostridium, Bacteroides, and Ruminococcus, which produce specialised enzymes capable of degrading the primary components of lignocellulose. These bacteria produce enzymes such as cellulases, xylanases, and ligninases, each acting on cellulose, hemicellulose, and lignin to break them down into simple sugars and other metabolites [64].
Recent metagenomic investigations of insect gut microbiomes have revealed that the makeup of these bacterial communities varies depending on diet, host species, and stage of development. Dietary factors strongly influence the gut microbiota, and specific plant-based diets, particularly those rich in lignocellulose, tend to promote a more diverse and specialised microbial population. For example, feeding Samia cynthia ricini various plant materials, including lignocellulose-rich leaves, boosts microbial diversity and improves the insect's capacity to digest diverse plant fibres [65]. The host's diet influences the enzymes produced by the gut microbiota. When exposed to higher concentrations of fibre or lignocellulosic material, the microbial community in the gut upregulates the production of cellulolytic and hemicellulolytic enzymes, thus improving the degradation of complex polysaccharides [66].
In addition to assisting digestion, gut bacteria may help detoxify toxic plant secondary metabolites. These metabolites, which are commonly used as plant defence mechanisms, can be poisonous to herbivores. The microbiota may break down these harmful substances, performing a detoxifying function that helps the insect host by allowing it to ingest a wider range of plants [67]. For example, certain bacteria in the gut microbiota are known to degrade phenolic compounds, which are abundant in many lignocellulosic plants, converting them into less harmful products [68].
Understanding the dynamics of gut microbiota in lignocellulose degradation brings up new possibilities for biotechnology, particularly biofuel production. Identifying new enzymes in these microbial communities opens the door to more efficient lignocellulose breakdown processes. Understanding the characteristics that influence microbial community composition, such as diet and host age, may also help to optimise these systems for industrial usage [69].
Functional gene studies in lignocellulose degradation
Functional gene investigations have shed light on the molecular processes behind lignocellulose breakdown in the gut microbiota of Saturniidae insects. Metagenomic and transcriptome investigations have found multiple genes that manufacture important enzymes, including cellulases, hemicellulases, and ligninases. These studies have revealed a broad repertoire of lignocellulolytic genes, which are often regulated by the type of plant material consumed by the host insect [70].
One important finding is the identification of genes encoding cellulases, including endoglucanase, exoglucanase, and β-glucosidase, which work together to hydrolyse cellulose into smaller sugars [71]. Similarly, genes that produce xylanases, arabinofuranosidases, and mannanases have been found to help break down hemicellulose [72]. Similarly, functional genes for laccases and peroxidases involved in lignin degradation have been discovered, revealing the gut microbiota's plasticity to degrade various components of lignocellulosic biomass [73]. These gene-based studies can guide attempts to improve enzyme production in industrial settings and are essential for comprehending the metabolic pathways involved in the breakdown of lignin and cellulose. Researchers may be able to increase the effectiveness of lignocellulose breakdown for biofuel generation by introducing these functional genes into model organisms or industrial microorganisms [74].
The role of insect gut microbiota in sustainable biofuel production
Saturniidae insects, particularly the Samia cynthia ricini (eri silkworm), have gut bacteria capable of degrading lignocellulosic biomass, providing a sustainable source of biofuels. Insect gut microbes are highly specialised for breaking down complex plant materials, and their enzymes can effectively break down cellulose, hemicellulose, and lignin into simple fermentable sugars to produce biofuels like ethanol, butanol, and methane [75].
The gut microbiota of Saturniidae insects offers several advantages for biofuel production. Because these insects consume a broad range of plants, including lignocellulose-rich ones, microbial consortia that can break down various biomass types can form. Second, gut microbes are excellent candidates for large-scale biofuel generation because they are very stable and active in adverse environments like high temperatures and pH fluctuations [76].
Researchers created artificial consortia that can efficiently break down lignocellulosic biomass in bioreactors by emulating the microbial community found in insect guts. Furthermore, insect-derived enzymes such as cellulases, xylanases, and ligninases have shown promise in increasing biofuels' production from lignocellulosic feedstocks [77]. The role of insect gut microbiota in biomass degradation might be necessary for developing sustainable biofuel technologies as second-generation biofuels gain popularity worldwide.
Enzymatic screening of gut bacteria
Enzymatic screening is critical in identifying gut bacterial isolates capable of efficiently degrading lignocellulosic biomass. Such screenings are designed to detect bacterial strains that produce highly active and stable enzymes essential for industrial-scale biofuel production. In Saturniidae insects, particularly the Samia cynthia ricini (eri silkworm), gut bacteria have been shown to produce a diverse array of cellulases, xylanases, and ligninases, which are capable of breaking down complex plant polymers [65].
For example, many studies have focused on identifying high-yielding cellulolytic strains. Clostridium species isolated from the gut of Samia cynthia ricini produce highly efficient cellulases capable of hydrolysing cellulose at high rates. At the same time, Cellulomonas strains are known for their ability to degrade both cellulose and hemicellulose [78]. Additionally, strains producing xylanases have been found to break down hemicellulose into simpler sugars like xylose, which can be fermented into biofuels. These enzymatic screenings help identify valuable microbial strains and provide insight into the optimal conditions for enzyme production, such as temperature, substrate and pH concentration. Since lignocellulosic feedstocks are often utilised in industrial settings, this information is essential for scaling up biofuel production methods [79].
Functional genomics and biotechnology for biofuel production
Functional genomics has completely transformed our knowledge of the microbial consortia involved in lignocellulose breakdown. By analysing the genetic makeup of gut microbiota from Saturniidae insects, researchers have identified key genes responsible for producing lignocellulolytic enzymes, such as cellulases, xylanases, and ligninases. These genes offer information on the molecular mechanisms behind the breakdown of biomass, which may be used to increase the processes involved in biofuel production [70].
These functional genes have been incorporated into model organisms thanks to recent developments in synthetic biology and metabolic engineering, producing genetically modified microorganisms that are more effective in breaking down lignocellulosic biomass. The ability to transfer multiple lignocellulolytic genes into a single microbial host could significantly increase the efficiency of biomass degradation, reducing the costs associated with biofuel production [64]. Furthermore, metagenomics and Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-Cas9 technology have enabled further optimisation of these microbes for industrial-scale biofuel production. The researchers want to build more efficient and sustainable biofuel production systems by combining functional genomics with bioreactor optimisation. These devices might dramatically reduce reliance on fossil fuels, providing a cleaner, renewable alternative [80].
Bioprospecting applications for biofuel production
Bioprospecting, or studying natural environments for beneficial microorganisms, is critical for discovering novel enzymes and microbial strains that can accelerate lignocellulose breakdown. By bioprospecting the stomach microbiota of Saturniidae insects, microbial strains with better enzymatic activity for decomposing complex plant components have been found [81]. Since effective lignocellulose de-radiation is crucial in transforming plant biomass into fermentable sugars, these findings significantly impact biofuel generation. In addition to identifying cellulolytic, xylanolytic, and ligninolytic enzymes, bioprospecting efforts aim to isolate bacteria that can thrive in harsh conditions such as high temperatures, acidic or alkaline environments, which are common in industrial biofuel production. This work can help improve the efficiency of biofuel production by providing microbes that are better suited for large-scale industrial applications [78].
Future prospects
Advances in microbial engineering
As research deepens into the genetic and metabolic traits of lignocellulose-degrading microbes from Saturniidae guts, opportunities will expand for engineering microbes with enhanced lignin-degrading capabilities. Through genetic modification, it may be possible to boost enzyme activity, improve substrate utilisation, and increase overall biofuel yields.
Metagenomics and transcriptomics
Employing next-generation sequencing technologies, such as metagenomic and metatranscriptomic analyses, can uncover insect gut communities' full microbial diversity and functional potential. Using these in termites, previously unknown lignocellulolytic enzymes will be found and characterised, opening the door to more specialised and effective biofuel production systems.
Formation of consortia of enzymes
The synergistic action of several enzymes is frequently necessary for the efficient breakdown of lignocellulose. Stronger and stable enzymatic formulations may arise from research into enzyme mixes obtained from Saturniidae gut bacteria. Optimising enzyme ratios, increasing heat and pH stability, and fine-tuning substrate specificity are all required stages in tailoring these cocktails for commercial application. Integrated bioprocessing systems: Integrating biofuel production into bigger biorefinery structures is another interesting development.
These systems are intended to generate biofuels and valuable bioproducts like organic acids, animal feed, and bioplastics. A complete and circular approach based on insect-derived microbiota has the potential to improve the economic and environmental sustainability of lignocellulosic bioconversion dramatically.
Environmental assessment and sustainability
A Comprehensive evaluation of the ecological impact of utilising insect gut microbes for biofuel production is essential. Future studies should include life cycle assessments to measure energy input, greenhouse gas emissions, and overall sustainability compared to traditional biofuel methods.
Industrial scalability and commercialisation
Translating laboratory-scale findings into commercially viable processes will be challenging. Reliable and scalable biofuel outputs will entail the creation of effective large-scale fermentation technologies, economical enzyme production techniques, and strong process optimisation.
CONCLUSION
The gut microbiota of Saturniidae insects such as Samia cynthia ricini, Antheraea assamensis, and Antheraea mylitta is a well-evolved microbial community capable of effectively digesting lignocellulosic biomass. These microbial communities contain a diverse spectrum of bacteria and fungi with cellulolytic and hemicellulolytic capabilities and important enzymes such as cellulases and xylanases, which are required to break down complex plant polymers into fermentable sugars. The teamwork among microbes boosts the digestive capabilities of their insect hosts and holds great promise for biotechnological uses, especially in creating cost-effective and sustainable biofuel systems. The ability of insect gut microbiota to adapt to dietary changes raises the prospect of creating specialised microbial systems that can break down particular lignocellulosic feedstocks. The digestive systems of insects in the Saturniidae family are an essential biological resource for discovering new enzymes and microbial candidates with industrial significance, connecting creative bioenergy solutions with natural ecological roles.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
Sheshu Munivenkatappa conceptualised and designed the review; conducted an extensive literature survey; drafted, wrote, and critically revised the manuscript; curated and interpreted data; and developed the graphical content and figures.
Malliah Shivashankar provided guidance in structuring the manuscript; contributed to data interpretation and critical evaluation of content; ensured clarity and consistency in scientific formatting; and reviewed and refined the final version of the manuscript for submission.
CONFLICT OF INTERESTS
Declared none
REFERENCES
Chukwuma OB, Obieze CC, Nwaiwu O, Okolo BN. Progress and perspectives of microbial degradation of lignocellulosic biomass for biofuel production. Renew Sustain Energy Rev. 2021;138:110566.
Statista. Global CO₂ emissions from fossil fuels from 1940 to 2024 (in billion metric tons); 2024. Available from: https://www.statista.communstat/264699/worldwide-co2emissions.
The Guardian. Global primary energy consumption by source (1950-2024). The Guardian; 2023. Available from: https://www.theguardian.com/environment/article/2024/jun/20/fossil‑fuel‑use‑reaches‑global‑record‑despite‑clean‑energy‑growth.
Manikandan S, Vickram S, Sirohi R, Subbaiya R, Krishnan RY, Karmegam N. Critical review of biochemical pathways to transformation of waste and biomass into bioenergy. Bioresour Technol. 2023 Mar;372:128679. doi: 10.1016/j.biortech.2023.128679, PMID 36706818.
Aidan Farrow, Greenpeace, Kathryn A, Miller, Greenpeace, Lauri Myllyvirta. Toxic air: the price of fossil fuels. Greenpeace; 2020. Available from: https://www.greenpeace.to/greenpeace/?p=3215.
Demirbas A. Political economic and environmental impacts of biofuels: a review. Appl Energy. 2009 Nov 1;86:S108-17. doi: 10.1016/j.apenergy.2009.04.036.
Nigam PS, Singh A. Production of liquid biofuels from renewable resources. Prog Energy Combust Sci. 2011;37(1):52-68. doi: 10.1016/j.pecs.2010.01.003.
Naik SN, Goud VV, Rout PK, Dalai AK. Production of first and second generation biofuels: a comprehensive review. Renew Sustain Energy Rev. 2010;14(2):578-97. doi: 10.1016/j.rser.2009.10.003.
Chaturvedi V, Verma P. An overview of key pretreatment processes employed for bioconversion of lignocellulosic biomass into biofuels and value-added products. 3 Biotech. 2013;3(5):415-31. doi: 10.1007/s13205-013-0167-8, PMID 28324338.
Rawat I, Ranjith Kumar RR, Mutanda T, Bux F. Biodiesel from microalgae: a critical evaluation from laboratory to large-scale production. Appl Energy. 2013;103:444-67. doi: 10.1016/j.apenergy.2012.10.004.
Georgianna DR, Mayfield SP. Exploiting diversity and synthetic biology for the production of algal biofuels. Nature. 2012;488(7411):329-35. doi: 10.1038/nature11479, PMID 22895338.
International Energy Agency (IEA). Renewables 2023: market report. IEA; 2023. Available from: https://www.iea.org/reports/technologies-to-make-biofuels-greener.
Karp A, Richter GM. Meeting the challenge of food and energy security. J Exp Bot. 2011;62(10):3263-71. doi: 10.1093/jxb/err099, PMID 21515638.
Ghosh A, Roy S, Das P, Banerjee R. Lignocellulolytic potential of insect gut microbiota for sustainable biofuel production: a case study on Saturniidae. J Microbiol Biotechnol. 2023;45(3):215-29.
Saha P, Das R, Chatterjee A, Mukherjee S. Role of gut microbiota in lignocellulose degradation and its potential for biofuel production in Saturniidae insects. Renew Bioenergy J. 2023;28(2):145-58.
Schwarz WH. The cellulosome and cellulose degradation by anaerobic bacteria. Appl Microbiol Biotechnol. 2001;56(5-6):634-49. doi: 10.1007/s002530100710, PMID 11601609.
Sakamoto M, Benno Y. Reclassification of bacteroides distasonis vulgatus and thetaiotaomicron. Int J Syst Evol Microbiol. 2006;56(7):1623-9.
Masai E, Katayama Y, Fukuda M. Genetic and biochemical investigations on bacterial catabolic pathways for lignin-derived aromatic compounds. Biosci Biotechnol Biochem. 2007;71(1):1-15. doi: 10.1271/bbb.60437, PMID 17213657.
Hammer TJ, Janzen DH, Hallwachs W, Jaffe SP, Fierer N. Caterpillars lack a resident gut microbiome. Proc Natl Acad Sci USA. 2017;114(36):9641-6. doi: 10.1073/pnas.1707186114, PMID 28830993.
Mazza G, Shoemaker P, Bouaid A. Lignocellulosic biomass for bioethanol production: advances and limitations. Biofuels Bioprod Biorefin. 2011;5(3):261-77.
Anand AA, Vennison SJ, Sankar SG, Prabhu DI, Vasan PT, Raghuraman T. Isolation and characterization of bacteria from the gut of Bombyx mori that degrade cellulose xylan pectin and starch and their impact on digestion. J Insect Sci. 2010;10(1):107. doi: 10.1673/031.010.10701, PMID 20874394.
Sengupta A, Roy D, Ghosh S. Microbial bioconversion of lignocellulosic biomass into biofuels: recent advances and prospects. Renew Sustain Energy Rev. 2021;135:110225.
Zhou J, Bruns MA, Tiedje JM. Environmental genomics and functional gene arrays in microbial ecology. Annu Rev Microbiol. 2010;64:451-77.
Rani A, Sharma A, Rajagopal R, Adak T, Bhatnagar RK. Bacterial diversity analysis of larvae and adult midgut microflora using culture-dependent and culture-independent methods in lab-reared and field-collected anopheles stephensi, an Asian malarial vector. BMC Microbiol. 2009;9:96. doi: 10.1186/1471-2180-9-96, PMID 19450290.
Rajagopal R, Sivakumar S, Agrawal N, Malhotra P, Bhatnagar RK, Rani A. Gut microbiota of insects: diversity and function in digestion of lignocellulosic biomass. Curr Sci. 2013;104(8):1034-40.
Debnath S, Saha D, Ghosh S. Gut microbial profiling of Samia cynthia ricin I reveals cellulolytic bacterial diversity for potential lignocellulose degradation. J Appl Microbiol. 2022;132(1):114-27.
Mason LM, Rubino F, Gozzi D, Manfredi M, Mandrioli M, Crotti E. Insect gut microbiota and their role in plant biomass degradation. Microb Ecol. 2015;70(2):257-71.
Beguin P, Aubert JP. The biological degradation of cellulose. FEMS Microbiol Rev. 1994;13(1):25-58. doi: 10.1111/j.1574-6976.1994.tb00033.x, PMID 8117466.
Barman J, Boro D, Goswami L, Talukdar NC. Diversity of gut microbiota in Antheraea assamensis a non-mulberry silkworm of North-East India. Microb Ecol. 2017;74(1):38-50.
Brune A. Symbiotic digestion of lignocellulose in termite guts. Nat Rev Microbiol. 2014;12(3):168-80. doi: 10.1038/nrmicro3182, PMID 24487819.
Sun Y, Cheng J. Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol. 2002;83(1):1-11. doi: 10.1016/s0960-8524(01)00212-7, PMID 12058826.
Chandel AK, Da Silva SS, Singh OV, Singh DP. Detoxification of lignocellulosic hydrolysates for improved bioethanol production. In: Biofuel production recent developments and prospects. Springer; 2012. p. 225-46.
Zhao X, Zhang L, Liu D. Biomass recalcitrance. Part I: the chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels Bioprod Bioref. 2012;6(4):465-82. doi: 10.1002/bbb.1331.
Van Dyk JS, Pletschke BI. A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes factors affecting enzymes conversion and synergy. Biotechnol Adv. 2012;30(6):1458-80. doi: 10.1016/j.biotechadv.2012.03.002, PMID 22445788.
Yuan TQ, Wang W, Xu F, Sun RC. Advanced NMR techniques characterise lignin structures and lignin carbohydrate complex (LCC) linkages. Materials (Basel). 2018;11(11):2043.
Liu ZH, Hao N, Xu J. Recent advances in understanding the microbial degradation of lignin. FEMS Microbiol Rev. 2020;44(2):123-44.
Cotana F, Cavalaglio G, Gelosia M, Coccia V, Petrozzi A, Pisello AL. Lignocellulosic biomass as a sustainable energy source: a review. J Renew Sustain Energy. 2014;6(1):013104.
Brune A. Symbiotic digestion of lignocellulose in termite guts. Nat Rev Microbiol. 2014;12(3):168-80. doi: 10.1038/nrmicro3182, PMID 24487819.
Engel P, Moran NA. The gut microbiota of insects diversity in structure and function. FEMS Microbiol Rev. 2013;37(5):699-735. doi: 10.1111/1574-6976.12025, PMID 23692388.
Xia Q, Li S, Fuan X. Gut microbiota in the Samia cynthia ricin I and its role in the metabolism of plant lignocellulose. FEMS Microbiol Ecol. 2013;85(2):336-48.
Rajan JV, Raghavendra A, Venkatesan A. Gut microbiota of Saturniidae insects and their potential in lignocellulose degradation. Microb Ecol. 2020;79(1):56-69.
Lynd LR, Weimer PJ, Van Zyl WH, Pretorius IS. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev. 2002;66(3):506-77. doi: 10.1128/MMBR.66.3.506-577.2002, PMID 12209002.
Singhania RR, Patel AK, Sukumaran RK, Larroche C, Pandey A. Role and significance of beta-glucosidases in the hydrolysis of cellulose for bioethanol production. Bioresour Technol. 2013 Jan;127:500-7. doi: 10.1016/j.biortech.2012.09.012, PMID 23069613.
Shallom D, Shoham Y. Microbial hemicellulases. Curr Opin Microbiol. 2003;6(3):219-28. doi: 10.1016/s1369-5274(03)00056-0, PMID 12831897.
Polizeli ML, Rizzatti AC, Monti R, Terenzi HF, Jorge JA, Amorim DS. Xylanases from fungi: properties and industrial applications. Appl Microbiol Biotechnol. 2005;67(5):577-91. doi: 10.1007/s00253-005-1904-7, PMID 15944805.
Boraston AB, Bolam DN, Gilbert HJ, Davies GJ. Carbohydrate binding modules: fine tuning polysaccharide recognition. Biochem J. 2004;382(3):769-81. doi: 10.1042/BJ20040892, PMID 15214846.
Wong DW. Structure and action mechanism of ligninolytic enzymes. Appl Biochem Biotechnol. 2009;157(2):174-209. doi: 10.1007/s12010-008-8279-z, PMID 18581264.
Saini A, Aggarwal NK, Sharma A, Yadav A. Actinomycetes: a source of lignocellulolytic enzymes. Enzyme Res. 2015;2015:279381. doi: 10.1155/2015/279381, PMID 26793393.
Janusz G, Pawlik A, Sulej J, Swiderska Burek U, Jarosz Wilkolazka A, Paszczynski A. Lignin degradation: microorganisms, enzymes involved genomes analysis and evolution. FEMS Microbiol Rev. 2017;41(6):941-62. doi: 10.1093/femsre/fux049, PMID 29088355.
Rogers PL, Jeon YJ, Lee KJ, Lawford HG. Zymomonas mobilis for fuel ethanol and higher-value products. Adv Biochem Eng Biotechnol. 2007;108:263-88. doi: 10.1007/10_2007_060, PMID 17522816.
Demain AL, Newcomb M, Wu JH. Cellulase, clostridia and ethanol. Microbiol Mol Biol Rev. 2005;69(1):124-54. doi: 10.1128/MMBR.69.1.124-154.2005, PMID 15755956.
Jeffries TW, Jin YS. Metabolic engineering for improved fermentation of pentoses by yeasts. Appl Microbiol Biotechnol. 2004;63(5):495-509. doi: 10.1007/s00253-003-1450-0, PMID 14595523.
Zuroff TR, Curtis WR. Developing symbiotic consortia for lignocellulosic biofuel production. Appl Microbiol Biotechnol. 2012;93(4):1423-35. doi: 10.1007/s00253-011-3762-9, PMID 22278256.
Bugg TD, Ahmad M, Hardiman EM, Singh R. The emerging role for bacteria in lignin degradation and bio-product formation. Curr Opin Biotechnol. 2011;22(3):394-400. doi: 10.1016/j.copbio.2010.10.009, PMID 21071202.
Stephanopoulos G. Challenges in engineering microbes for biofuels production. Science. 2007;315(5813):801-4. doi: 10.1126/science.1139612, PMID 17289987.
Keasling JD. Manufacturing molecules through metabolic engineering. Science. 2010;330(6009):1355-8. doi: 10.1126/science.1193990, PMID 21127247.
Sanchez OJ, Cardona CA. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour Technol. 2008;99(13):5270-95. doi: 10.1016/j.biortech.2007.11.013, PMID 18158236.
Jani RP, Rajan JV, Patel MR. Characterisation and applications of lignocellulolytic enzymes from insect gut microbiota. Environ Sci Pollut Res Int. 2019;26:24879-91.
Singh GB, Zhang Y, Boini KM, Koka S. High mobility group box 1 mediates TMAO-induced endothelial dysfunction. Int J Mol Sci. 2019;20(14):3570. doi: 10.3390/ijms20143570, PMID 31336567.
Rajan JV, Raghavendra A, Venkatesan A. Gut microbiota of saturniidae insects and their potential in lignocellulose degradation. Microb Ecol. 2020;79(1):56-69.
Wei C, Zhang X, Wei H. Screening and characterisation of lignocellulolytic enzymes from gut bacteria of herbivorous insects. Appl Microbiol Biotechnol. 2020;104(12):5447-56.
Campanile A, Liguori B, Marino O, Cavaliere G, De Bartolomeis VL, Caputo D. Facile synthesis of nanostructured cobalt pigments by Co-a zeolite thermal conversion and its application in porcelain manufacture. Sci Rep. 2020;10(1):10147. doi: 10.1038/s41598-020-67282-1, PMID 32576904.
Rajan A, Rajan A, Gaur R, Sharma R. Microbial diversity in the gut of eri silkworm Samia cynthia ricin I: a potential source of lignocelluloses degrading microbes. Biocatal Agric Biotechnol. 2020;29:101799.
Li X, Zhao C, Zhang X. Characterisation of lignocellulolytic enzyme-producing bacteria in the gut microbiota of the eri silkworm, Samia cynthia ricin I. Appl Microbiol Biotechnol. 2016;100(6):2575-84.
Xia W, Li X, Sun J. Microbial enzymes for lignocellulose degradation and their applications in biofuel production. Appl Microbiol Biotechnol. 2013;97(16):7223-31.
Yu T, Wang Y, Chen S, Hu M, Wang Z, Wu G. Low molecular weight chitosan supplementation increases the population of Prevotella in the cecal contents of weanling pigs. Front Microbiol. 2017;8:2182. doi: 10.3389/fmicb.2017.02182, PMID 29163454.
Zhou X, Zhang K, Zhang T, Yang Y, Ye M, Pan R. Formation of odorant haloanisoles and variation of microorganisms during microbial O-methylation in annular reactors equipped with different coupon materials. Sci Total Environ. 2019;679:1-11. doi: 10.1016/j.scitotenv.2019.04.329, PMID 31078770.
Jiang Y, Xie M, Zhang H. Gut microbiota of herbivorous insects and its potential role in biomass degradation. Appl Environ Microbiol. 2020;86(15):e01115-20.
Zhu J, Wang X, He X. The symbiotic relationship between gut microbiota and Samia cynthia ricin I in lignocellulose degradation. Biochem Biophys Res Commun. 2017;491(3):704-10.
Cheng G, Xu Z, Zhang Y. Functional genomics of lignocellulolytic enzymes in gut microbiota of herbivorous insects. FEMS Microbiol Lett. 2017;364(9):fnx081.
Yi X, Gao Q, Zhang L, Wang X, He Y, Hu F. Heterozygous diploid structure of Amorphotheca resinae ZN1 contributes efficient biodetoxification on solid pretreated corn stover. Biotechnol Biofuels. 2019;12:126. doi: 10.1186/s13068-019-1466-z, PMID 31139256.
Miller SE, Zhang Q, Griffiths MW. Microbial xylanases from insect gut microbiota: characterisation and application. Enzyme Microb Technol. 2016;85:89-96.
Zhao L, Zhang Y, Cheng X. Lignin degradation by insect gut microbiota and its implications for biofuel production. Appl Microbiol Biotechnol. 2018;102(9):3891-901.
Elia R, Sanz JL PC. Functional genomics of lignocellulose degradation in microbial consortia: from lab to industry. Appl Microbiol Biotechnol. 2020;104(4):1393-408.
Zhong J, Chen D, Zhu HJ, Gao BD, Zhou Q. Hypovirulence of sclerotium rolfsii caused by associated RNA mycovirus. Front Microbiol. 2016;7:1798. doi: 10.3389/fmicb.2016.01798, PMID 27891121.
Hong PY, Liu Z, Liu X. Role of insect gut microbiota in lignocellulose degradation and biofuel production. Environ Sci Technol. 2015;49(15):9269-77.
Singh S, Singh O, Verma A. Enzymatic deconstruction of lignocellulosic biomass for biofuels production. Bioenergy Res. 2014;7(2):148-57.
Darton TC, Baker S, Randall A, Dongol S, Karkey A, Voysey M. Identification of novel serodiagnostic signatures of typhoid fever using a salmonella proteome array. Front Microbiol. 2017;8:1794. doi: 10.3389/fmicb.2017.01794, PMID 28970824.
Matle I, Mafuna T, Madoroba E, Mbatha KR, Magwedere K, Pierneef R. Population structure of non-ST6 Listeria monocytogenes isolated in the red meat and poultry value chain in South Africa. Microorganisms. 2020;8(8):1152. doi: 10.3390/microorganisms8081152, PMID 32751410.
Sun X, Yang Z, Hong X, Zaslawski S, Wang S, Soto MA. Genetic optimised aperiodic code for distributed optical fibre sensors. Nat Commun. 2020;11(1):5774. doi: 10.1038/s41467-020-19201-1, PMID 33188171.
Hacquard S. Commentary: microbial small talk: volatiles in fungal bacterial interactions. Front Microbiol. 2017;8:1. doi: 10.3389/fmicb.2017.00001, PMID 28197127.