
Int J Chem Res, Vol 9, Issue 4, 34-39Research Article
SYNTHESIS AND EVALUATION OF DIAZO-TRIAZOLE HYBRID AS ANTI-TUBERCULAR AGENTS
DINESH KACHKUREa, SACHIN A. DHAWALEc, GANESH TAPADIYAc, CHANDRAKANT PAWARb, JAGDISH BHARADa*
aDepartment of Chemistry, Vasantrao NaikMahavidyalaya, Aurangabad-431004, Maharashtra, India. bDepartment of Chemistry, Deogiri College, Aurangabad-431005, Maharashtra, India. cDepartment of Pharmaceutical Chemistry, Shreeyash Institute of Pharmaceutical Education and Research, Aurangabad-431010, Maharashtra, India
*Corresponding author: Jagdish Bharad; *Email: [email protected]
Received: 05 Jul 2025 Revised and Accepted: 25 Aug 2025
ABSTRACT
Objective: To find novel anti-tubercular agents by using diazo-triazole hybrid. A series of new(E)-4-((2,4-dichloro-3-((substituted)diazenyl)phenoxy)methyl)-1-(substituted)-1H’-1,2,3-triazole derivatives (6a-6m) were synthesized from readily available anilines (1a-1d) and 2, 4-dichlorophenol (2). A total of 21 novel diazenyl-triazole derivatives were synthesized. Diazotization and click reactions were used in a sequence of processes to create the targets.
Methods: Merck and Sigma-Aldrich provided analytical grade (AR) chemicals and reagents, which were employed directly without further purification. All FT-IR spectra were recorded using a Bruker Alpha FT-IR spectrophotometer (Billerica, MA, USA) with the universal ATR sampling attachment. The 1H, 13C, and 19F NMR spectra of all synthesized compounds were recorded.
Results: The synthesized derivatives are characterized by FT-IR, 1H NMR, 19F, and 13C NMR analytical techniques. The anti-tubercular (Mycobacterium tuberculosis: H37Rv strain) properties of the novel compounds were evaluated in vitro. Many compounds exhibited moderate to good activity. The compounds 3a, 3b, 3d and 6i showed good inhibition (MIC = 6.25-12.5 µm) against H37Rv strain.
Conclusion: The synthesized compounds exhibited good antimicrobial activity and can serve as potent agent against antimicrobial agents. Other derivatives showed moderate to lower inhibition for all the strains.
Keywords: Anti-tubercular, Diazenyl, Triazole, Diazotization, Click
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/)
DOI: http://dx.doi.org/10.22159/ijcr.2025v9i4.281 Journal homepage: https://ijcr.info/index.php/journal
INTRODUCTION
Heterocyclic drug discovery research is an ongoing activity for a variety of legitimate reasons, including drug resistance, medication expense, treatment duration, and therapeutic ineffectiveness, among many others. Since humans began using pharmaceuticals in our early years, new strains of microbes that are resistant to the drugs we use have emerged. Therefore, the creation of better and more effective medications is always needed [1]. Mycobacterium tuberculosis (Mtb) is the causative agent of TB, a contagious disease spread through the air. The World Health Organization recorded 8.6 million illnesses in 2012, with 1.3 million deaths among those cases. In 2016, an estimated 490000 new instances of multidrug resistance were reported [2]. 10.4 million new cases of Mycobacterium tuberculosis (Mtb) are reported each year, with an average of 1.7 million fatalities [3]. Drug resistance is increasing, making medications more lethal and challenging to treat. The diseases known as multidrug resistance (MDR) and extensive drug resistance (XDR) Mtb are brought on by germs that are resistant to first-line anti-tubercular medications. The current course of treatment involves taking a variety of medications for years at a time, which has many negative effects and is very expensive. Since Mtb can be acquired through the air, pollution is the biggest issue in developing nations. Although the Although the death rate has lately dropped, it is still the second most common cause of death, after AIDS. To address resistance to the first-and second-line medications already on the market, Mtb remains the focus of numerous studies [4]. The majority of medications used to treat MTB, including as isoniazid, pyrazinamide, ethambutol, and rifampicin, were created 60–70 years ago. More recently, bedaquiline, delamanid, and pretomanid have been made accessible on the market [5, 6]. Due to their high cost and limited application to multidrug-resistant Mtb (MDR-Tb), the newly discovered compounds are not generally employed [7, 8]. These recently created compounds lack hydrogen bond donors and have the highest hydrophobicity (A Log P) when compared to previous medications [9]. According to the WHO, Mtbcan reoccur as a latent disease, based on the immune system of the patient, so research on TB drugs will remain a major target for researchers [10].
Azoles are the most important class of nitrogen heterocycle, which shows various biological activities like anti-malarial, anti-fungal, antimicrobial, anti-HIV, anti-inflammatory, anti-TB, etc. The 1,2,3-triazole shows various biological activities like anti-HIV, antibiotics, antibacterial, etc. The triazoles framework shows properties like moderate dipole character, easily hydrogen bonding capability, rigidity, stability under in vivo conditions, it inhibits the growth of bacteria by blocking lipid biosynthesis [11]. With several biological uses, including antibacterial, antihistaminic, anti-inflammatory, antimalarial, antiallergic, anti-HIV, anti-diabetic, anti-tubercular, anti-parasitic, anti-obesity, antihypertensive, anti-cancer, and antimicrobial properties, the 1,2,3-triazole was a significant class of scaffold [12-14]. The activity of triazole nuclei is due to its isostere with imidazole, oxazole, pyrazole, thiazole etc. The triazole shows promising pharmacological activity, low toxicity, less adverse effects, higher bioavailability, good pharmacokinetic properties, diversity of drug administration, broad spectrum of activities etc. [15]. The 1,3-dipolar cycloaddition reaction of 1,3-dipole to dipolarophile for the synthesis of five five-membered heterocycle is well known as Click reaction in organic chemistry. The Cu(1) catalyst is a versatile catalyst for the synthesis of 1,4-disubstittuted-1,2,3-triazole [16-19].
The researches on diazenyl compounds are very ancient. It found its applications in medicinal chemistry, material chemistry, dyes, polymers, lasers, agricultural field as pesticides etc. The diazenyl compound founds applications in electro-optical properties [20-24].
From literature reviews the different combinations of triazole derivatives shows varied biological activities. By considering this fact we coupled the triazole nuclei with diazenyl nuclei in one pharmacophore to bet better biological activities. These synthetic derivatives were further studied for their anti-tubercular activities against H37Rv strain. In an attempt to increase biological activity, we create and synthesize these targets based on the diverse actions of various groups, both individually and collectively.
The most popular method for creating novel derivatives with a combination of active pharmacophores is molecular hybridization. The alternate strategy was to take advantage from the available literature and develop new targets by keeping active core constant. We used both the approaches as we chosen the 1,2,3-triazole nuclei and diazenyl nuclei by considering their diversified role in biological activities. For designing we have taken the advantage of available drugs containing both diazenyl nuclei and triazole nuclei as depicted in fig. 1 below. Inflammatory bowel illness was treated with the anti-inflammatory medication balsalazide. The medication sulfasalazine was used as a first-line treatment for Crohn's disease, ulcerative colitis, and rheumatoid arthritis. Ulcerative colitis and other inflammatory bowel disorders were treated with the anti-inflammatory medication olsalazine. In addition to having diazenyl nuclei, the compound NSC-83318 exhibits promising anti-mycobacterial activity against M. bovis BCG and INH-susceptible and INH-resistant M. tuberculosis H37Rv. Compared to standard isoniazid, the minimum inhibitory concentration values for M. bovis were 0.39 µg/ml, Mtb H37Rv was 12.5 µg/ml, and Mtb H37RvINH was 12.5 µg/ml[25]. These three are the marketed drugs for the treatment of different diseases all belong to diazenyl nuclei family.
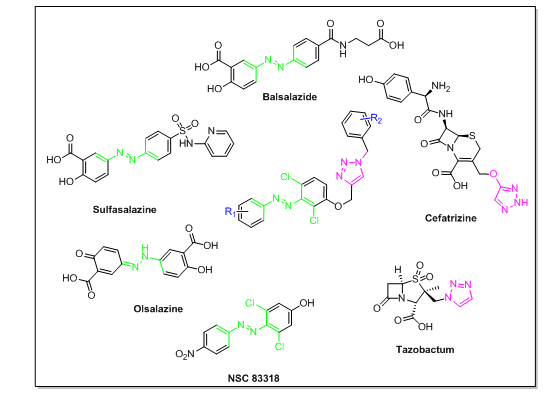
Fig. 1: Structures of some of the available drugs bearing diazenyl and triazole
The drugs Tazobactum and Cefatrazine were used for antimicrobial drugs. These drugs were used to treat the bacterial infections by inhibiting the action of bacteria β–lactamases. The drug Cefatrazine was used as a broad-spectrum cephalosporin anti-biotics, it is used for the treatment of both g-positive and negative bacterial infections.
By considering the diversified roles of marketed drugs belonging to diazenyl and 1,2,3-triazole scaffolds we designed the combination of triazole nuclei along with diazenyl through ether linkage to get better biological activity.
MATERIALS AND METHODS
Merck and Sigma-Aldrich provided analytical grade (AR) chemicals and reagents, which were employed directly without further purification. Thin Layer Chromatography (TLC) on precoated silica gel 60 F254 (mesh) (E. Merck) was used to monitor the progress of the reactions and the purity of the produced compounds, with spots visible under UV light (long and short wavelengths). Merck silica gel (60-120 mesh) was used in column chromatography. Melting points of all synthesized compounds were measured in open capillaries using Thermo Fisher Scientific's (IA9000, UK) digital melting point instrument and are uncorrected. All FT-IR spectra were recorded using a Bruker Alpha FT-IR spectrophotometer (Billerica, MA, USA) with the universal ATR sampling attachment. The 1H, 13C, and 19F NMR spectra of all synthesized compounds were recorded on a Bruker Avance IV NMR spectrometer at 400 and 100 MHz using CDCl3 or DMSO d6, using TMS as an internal standard, and signals were reported in parts per million (ppm). All high-resolution mass spectra (HRMS) were collected using an Autospec mass spectrometer with electron spray ionization [26].
RESULTS AND DISCUSSION
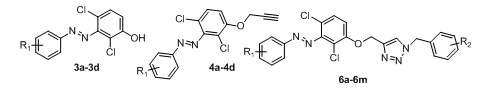
A sequence of processes, including diazotization, O-alkylation, and the click reaction, were used to synthesize substituted diazo triazole derivatives (6a-6m). The final derivatives were synthesized by optimizing the reaction stages, considering factors such as yield, reaction time, raw material and reagent costs, less hazardous circumstances, and neat reaction profiles. Column chromatography and, occasionally, combi-flash are used to purify each derivative. Scheme 1 below shows the specific chemical sequences for the synthesis of 6a-6m.
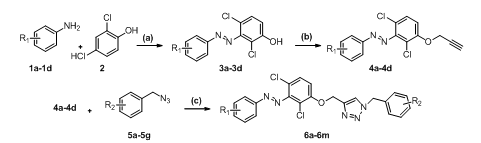
Scheme 1: Synthesis of 2-(substituted-diaza triazole derivatives (6a-6m)
Reagents and conditions
(a):NaNO2, HCl, NaOH, H2O,-10 °C, 4h; (b): Propargyl bromide, K2CO3, TBAI, acetone, 70 °C, 3h; (c): CuSO45H2O, sodium ascorbate, t-BuOH, H2O, rt, 18 h.
In step a, we have done Diazotization reaction by using different anilines 1a-1d and 2, 4-dichloro phenol (2) to obtain intermediate 3a-3d. Previous research shows different acidic conditions were used for diazotization reactions. We have optimized step a, for betting better reaction condition with the concern of safety, yield, time and economy of reactions. For model reaction we have used 3-Nitro aniline (1a) as a model substrate for optimization purpose. We have used hydrochloric acid and sulfuric acid for making diazo-halide. We have used NaOH and KOH as bases for the reaction and reaction was done in water. The detailed results are summarized below after scheme 2.
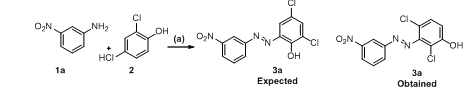
Scheme 2: Synthesis of (E)-2,4-dichloro-3-((3-nitrophenyl)diazenyl)phenol (3a)
Reagents and conditions: (a): NaNO2, HCl, NaOH, H2O,-10 °C, 4h.
In first optimization reaction we have used 6N aq. HCl for diazotization along with NaOH and KOH as bases at-10 °C to obtain desired compound with 90% and 65% yield. Interestingly we obtain 3-nitrophenyl diazaneyl derivative instead of 6-nitrophenyl diazeneyl derivative. The electronic effect to chlorine was more compared to electronic effect of hydroxyl group in the product 3a. Further we have done reactions with stronger acid sulfuric acid and by using NaOH and KOH as bases, both the reactions produces unexpected compound 3a with 65% and 55% yields at-10 °C. The desired compound structure was confirmed by the help of 1H NMR as the phenol ring should show two singlets for aromatic protons at δ 7.64 and δ 7.17, but instead it's showing doublet at δ7.78 and δ7.65 ppm confirming the unexpected product obtained. The results further confirmed by their 13C NMR spectrum. The base NaOH was the best for the diazotization reaction as with KOH, some non-polar impurities ware formed which results in a decrease in product yields. The HCl was preferred over sulfuric acid as the obtained yield from prior is better than the latter.
Using propargyl bromide and base under various circumstances, we performed O-alkylation in step b. Propargyl ethers were synthesized using a variety of bases and conditions, according to earlier studies. As bases, we employed K2CO3 and Cs2CO3, and as solvents, we used acetone and DMF. Compound 3a has been utilized as a model substrate for the O-alkylation process. Every reaction was carried out under heating and room temperature settings, and table 1 lists the outcomes of the optimization reactions.
Table 1: The optimization of base, solvent, temperature and time for the synthesis of (E)-1-(2,6-dichloro-3-(prop-2-yn-1-yloxy)phenyl)-2-(3-nitrophenyl)diazene (4a)
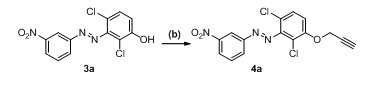
Reagents and conditions: (b): Propargyl bromide (1.5 eq.)
| Entry | Base/Additive | Solvent | Time (h) | Temperature (°C) | Yielda (%) |
| 1 | K2CO3 (2 eq.), TBAI (0.2 eq.) | DMF | 36 | RT | 55 |
| 2 | K2CO3 (2 eq.) | DMF | 10 | 120 | 60 |
| 3 | K2CO3 (2 eq.), TBAI (0.2 eq.) | DMF | 18 | 60 | 55 |
| 4 | K2CO3 (2 eq.) | Acetone | 24 | RT | 45 |
| 5 | K2CO3 (2 eq.), TBAI (0.2 eq.) | Acetone | 3 | 70 | 90 |
| 6 | Cs2CO3 (2 eq.), TBAI (0.2 eq.) | DMF | 30 | RT | 50 |
| 7 | Cs2CO3 (2 eq.) | DMF | 12 | 120 | 55 |
| 8 | Cs2CO3 (2 eq.), TBAI (0.2 eq.) | DMF | 18 | 60 | 60 |
| 9 | Cs2CO3 (2 eq.), TBAI (0.2 eq.) | Acetone | 30 | RT | 60 |
| 10 | Cs2CO3 (2 eq.), TBAI (0.2 eq.) | Acetone | 6 | 70 | 70 |
| aIsolated Yields, RT-room temperature |
The results of the optimization reaction indicate the reaction in acetone at 70 °C gave 90% of desired product in entry 5 after using K2CO3 as base and TBAI as additive. The reaction with K2CO3 and Cs2CO3 at room temperature, (entry 1 and 6) after longer reaction hours of 30 h the starting material was not consumed and the product formation was in the range of 50% only. Further we have heated the reaction ata higher temperature 120 °C for 12h (entry 2 and 7) to get compound 4a with 55-60% yields. The higher temperature doesn’t help to increase the yield of the obtained product, as there is a chance of evaporation of propargyl bromide during reaction, as there is complete consumption of starting material 3a was not observed. After this observation we reduced the reaction temperature and monitored reaction progress (entry 3 and 8), after 18h the starting material was not consumed and there is 60% formation of 4a. Further we done the reaction by changing the solvent to acetone at room temperature (entry 4 and 9) after longer reaction hours there is 45% and 60% formation of 4a was observed. To reduce the time of reaction we heated reactions at 70 °C, (entry 5 and 10), after 3 h there is complete consumption starting material in K2CO3 reaction, after isolation, we got 90% of 4a. In the CS2CO3 reaction after 6h there is consumption of starting material but the isolated yield is 70%. The reaction time was reduced considerably and the formation of product to quantitative. The polarity of solvent temperature and time plays critical parameters for O-alkylation reactions to get the intermediate 4a. The compound was characterized by 1H NMR as there is a disappearance of OH peak from starting material and clear peak for –CH2 and acetylene was seen in 1H NMR spectra of 4a.
In step c, we have done synthesis of compound (6a-6m)1,4-disubstituted-1,2,3-triazoles efficiently prepared via a Click reaction. The compound 4a was taken along with1-(azidomethyl)-3-chlorobenzene (5a) as a model substrate for copper-catalyzed 1,3 dipolar cyclo-addition reactions. We have done two reactions by using copper diacetate [Cu (OAc)2], and copper sulfate pentahydrate [ CuSO4-5H2O] as catalysts in t-Butanol and THF as solvents. The reaction of copper diacetate in t-BuOH and THF gave 60% and 55% yield of product, respectively, after 18h. The same in reaction was done with copper sulfate pentahydrate in t-BuOH and THF to obtain 75% and 65% of product 6a, respectively. The desired product was confirmed by the disappearance of the acetylene peak of starting material. The 1H NMR of 6a shows clear singlet peaks at δ 5.54 and δ 5.52 for two –CH2, along with triazole –CH peak as singlet was observed at δ 8.59. The same procedure was applied for the synthesis of remaining derivatives.
The detailed experimental details and spectral data ware incorporated in the supporting information. The different substituents and their anti-tubercular activity results were summarized in below table 2.
Table 2: In vitro anti-tubercular activity against MTB H37Ra activity of compounds (3a-3d, 4a-4d and 6a-6m)
| Comp. No. | R1 | R2 | Yielda (%) | MP(°C) | M. tuberculosis H37 Rv, MIC (µg/ml) |
| 3a | 3-NO2 | - | 90 | 158-159 | 6.25-12.5 |
| 3b | 3-F | - | 95 | 163-165 | 6.25-12.5 |
| 3c | 3-CF3,4-Cl | - | 85 | 187-189 | >50 |
| 3d | 3-NO2,2-Cl | - | 83 | 195-197 | 12.5 |
| 4a | 3-NO2 | - | 90 | 137-139 | >50 |
| 4b | 3-F | - | 86 | 116-118 | >50 |
| 4c | 3-CF3,4-Cl | - | 90 | 132-134 | >50 |
| 4d | 3-NO2,2-Cl | - | 78 | 147-148 | >50 |
| 6a | 3-NO2 | 3-Cl | 75 | 179-181 | >50 |
| 6b | 3-NO2 | 3-F | 79 | 159-160 | >50 |
| 6c | 3-NO2 | 4-NO2 | 83 | 164-166 | >50 |
| 6d | 3-NO2 | 4-CH3 | 75 | 197-199 | >50 |
| 6e | 3-CF3,4-Cl | C2H4-OH | 55 | 114-116 | >50 |
| 6f | 3-CF3,4-Cl | C3H6-OH | 60 | 108-110 | >50 |
| 6g | 3-CF3,4-Cl | H | 80 | 201-203 | >50 |
| 6h | 3-CF3,4-Cl | 4-F | 70 | 198-200 | >50 |
| 6i | 3-F | 3-F | 75 | 188-190 | >50 |
| 6j | 3-F | H | 82 | 176-178 | >50 |
| 6k | 3-F | C2H4-OH | 65 | 116-118 | 12.5-25 |
| 6l | 3-F | C3H6-OH | 55 | 118-121 | >50 |
| 6m | 3-F | 4-NO2 | 75 | 202-204 | >50 |
| INH | 0.39 | ||||
| aIsolated Yields, MP-Melting Point |
Biological activity
From the anti-tubercular activity data from table 2 the SAR can be derived as the compounds having only R1 ring attached with nitro derivative at meta position is more active compared to other derivatives. The strong electron-withdrawing compounds shows promising activity against tested strains. The ring with flouro substitution at the meta position shows equivalent activity with meta nitro substituents. The phenolic OH in the ring plays a key role in the biological activity strongly. The electron effect of flouro and nitro are almost equal on the aromatic ring resulting in similar anti-tubercular activity. The compound 3a, 3b and 6k shows the potent antitubercular activity. The strong electron-withdrawing groups favors the promising antitubercular activity. The remaining derivatives 4a-4d and 6a-6m were moderately active compared with the standard [29]. Rajubai D Bakale et al. studied the assessment of 1, 2, 3-triazole incorporated thiazolylcarboxylate derivatives as antitubercular agents. They have designed library of compounds for their in vitro antitubercular activity against Mycobacterium tuberculosis H37Rv. The two compounds 7h and 8h have displayed excellent antitubercular activity with MIC values of 3.12 and 1.56 µg/ml respectively (MIC values of standard drugs; Ciprofloxacin 1.56 μg/ml and Ethambutol 3.12 μg/ml). Whereas, the four compounds 7i, 7n, 7p and 8i displayed noticeable antitubercular activity with a MIC value of 6.25 µg/ml. Our synthesized compounds also containing triazole with their hybrid showing comparable activity with the given standard and study done by Rajubai D Bakale et al. [30].
CONCLUSION
A novel diazenyl coupled (1,2,3)-triazole derivatives were synthesized via an easy and continent synthetic protocol from readily available anilines and 2,4-dichloro phenol. A novel 21 analogues 3a-3d, 4a-4d and 6a-6m ware synthesized using click reaction in three-step synthetic sequences. We standardize the reaction condition for diazotization, O-alkylation reaction and click reaction for getting cleaner, near reaction profile without much effort. All the synthesized derivatives ware characterized by NMR spectral data. These derivatives ware tested for their in vitro anti-tubercular activity against H37Rv. The compounds 3a,3b and 6k shows good inhibition compared to standard. In future need to synthesize more derivatives and test them for the possible good activity.
ACKNOWLEDGEMENT
Authors thankful to Head of Deogari collage, Aurangabad for providing the necessary facilities.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
Dinesh Kachkure-Original draft writing, Sachin A. Dhawale-editing and formatting, Ganesh Tapadiya-Methodology, Chandrakant Pawar-Supervision and editing, Jagdish Bharad-original concept.
CONFLICT OF INTERESTS
Declared none
REFERENCES
Morens DM, Folkers GK, Fauci AS. The challenge of emerging and re-emerging infectious diseases. Nature. 2004 Jul 8;430(6996):242-9. doi: 10.1038/nature02759, PMID 15241422.
World Health Organization. Global Tuberculosis Report. Geneva: World Health Organization; 2015. Available from: http://www.who.int/tb/country/en. [Last accessed on 15 May 2025].
World Health Organization. Global tuberculosis report. In: Geneva: World Health Organization; 2018. Available https://www.who.int/tb/publication/global_report/en. [Last accessed on 15 May 2025].
Jakab Z, Acosta CD, Kluge HH, Dara M. Consolidated action plan to prevent and combat multidrug and extensively drug resistant tuberculosis in the WHO European Region 2011-2015: cost-effectiveness analysis. Tuberculosis (Edinb). 2015;95Suppl 1:S212-6. doi: 10.1016/j.tube.2015.02.027, PMID 25829287.
Mikusova K, Ekins S. Learning from the past for TB drug discovery in the future. Drug Discov Today. 2017 Apr;22(3):534-45. doi: 10.1016/j.drudis.2016.09.025, PMID 27717850.
Cole ST. Tuberculosis drug discovery needs public-private consortia. Drug Discov Today. 2017;22(3):477-8. doi: 10.1016/j.drudis.2016.09.026, PMID 27717851.
Conradie F, Diacon AH, Ngubane N, Howell P, Everitt D, Crook AM. Treatment of highly drug-resistant pulmonary tuberculosis. N Engl J Med. 2020 Mar 5;382(10):893-902. doi: 10.1056/NEJMoa1901814, PMID 32130813.
Thwaites G, Nahid P. Triumph and tragedy of 21st century tuberculosis drug development. N Engl J Med. 2020 Mar 5;382(10):959-60. doi: 10.1056/NEJMe2000860, PMID 32130819.
Makarov V, Salina E, Reynolds RC, Kyaw Zin PP, Ekins S. Molecule property analyses of active compounds for Mycobacterium tuberculosis. J Med Chem. 2020;63(17):8917-55. doi: 10.1021/acs.jmedchem.9b02075, PMID 32259446.
Iacobino A, Piccaro G, Giannoni F, Mustazzolu A, Fattorini L. Tablehting tuberculosis by drugs targeting nonreplicating mycobacterium tuberculosis bacilli. Int J Mycobacteriol. 2017 Sep;6(3):213-21. doi: 10.4103/ijmy.ijmy_85_17, PMID 28776518.
Zhang S, Xu Z, Gao C, Ren QC, Chang L, LV ZS. Triazole derivatives and their anti-tubercular activity. Eur J Med Chem. 2017 Jul 20;138:501-13. doi: 10.1016/j.ejmech.2017.06.051, PMID 28692915.
Thirumurugan P, Matosiuk D, Jozwiak K. Click chemistry for drug development and diverse chemical biology applications. Chem Rev. 2013 Sep 11;113(7):4905-79. doi: 10.1021/cr200409f, PMID 23531040.
Xu Z, Song XF, Hu YQ, Qiang M, LV ZS. Azide alkyne cycloaddition towards 1H-1,2,3-triazole-tethered gatifloxacin and isatin conjugates: design synthesis and in vitro anti-mycobacterial evaluation. Eur J Med Chem. 2017 Jul 20;138:66-71. doi: 10.1016/j.ejmech.2017.05.057, PMID 28646656.
Thomas KD, Adhikari AV, Chowdhury IH, Sumesh E, Pal NK. New quinolin-4-yl-1,2,3-triazoles carrying amides, sulphonamides and amidopiperazines as potent anti-tubercular agents. Eur J Med Chem. 2011 Jul;46(6):2503-12. doi: 10.1016/j.ejmech.2011.03.039.
Zhou CH, Wang Y. Recent researches in triazole compounds as medicinal drugs. Curr Med Chem. 2012;19(2):239-80. doi: 10.2174/092986712803414213, PMID 22320301.
Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem Int Ed Engl. 2002;41(14):2596-9. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4, PMID 12203546.
Kolb HC, Finn MG, Sharpless KB. Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed Engl. 2001;40(11):2004-21. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5, PMID 11433435.
Boechat N, Ferreira VF, Ferreira SB, De Lourdes G Ferreira M, De C Da Silva F, Bastos MM. Novel 1,2,3-triazole derivatives for use against Mycobacterium tuberculosis H37Rv (ATCC 27294) strain. J Med Chem. 2011;54(17):5988-99. doi: 10.1021/jm2003624, PMID 21776985.
Dheer D, Singh V, Shankar R. Medicinal attributes of 1,2,3-triazoles: current developments. Bioorg Chem. 2017 Apr;71:30-54. doi: 10.1016/j.bioorg.2017.01.010, PMID 28126288.
Awad IM, Aly AA, Abdel RA, Ahmed SH. Synthesis and characterization of some new transition metal complexes. J Inorg Biochem. 1998;33:77-89.
Phillips OA, Udo EE, Abdel Hamid ME, Varghese R. Synthesis and antimicrobial activity of some triazole derivatives. Eur J Med Chem. 2009 Sep;44(9):3217-27.
Funar Timofei S, Fabian WM, Kurunczi L, Goodarzi M, Ali ST, Heyden YV. Modelling heterocyclic azo dye affinities for cellulose fibres by computational approaches. Dyes Pigm. 2012;94(2):278-89. doi: 10.1016/j.dyepig.2012.01.015.
Singh H, Sindhu J, Khurana JM, Sharma C, Aneja KR. Syntheses, biological evaluation and photophysical studies of novel 1,2,3-triazole-linked azo dyes. RSC Adv. 2014;4(12):5915-25. doi: 10.1039/c3ra44314k.
Selvarani S, Rajakumar P. Synthesis photochemical, electrochemical and cytotoxic studies on azobenzene-cored dendrimer decorated with chalcone motif. Chemistry Select. 2018 Oct;3(19):5455-60. doi: 10.1002/slct.201800286.
Kidwai S, Park CY, Mawatwal S, Tiwari P, Jung MG, Gosain TP. Dual mechanism of action of 5-nitro-1,10-phenanthroline against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2017 Aug;61(11):e00969-17. doi: 10.1128/AAC.00969-17, PMID 28893784.
Elzupir AO, Saeed AE, Barakat IE, Westhuizen JH. Ultrasound-assisted microwave synthesis and mechanistic aspect of 2-amino-4,6-diaryl pyrimidines and 3, 5-diaryl-1H-pyrazoles. Int J Curr Pharm Res. 2015 Jan;7(1):7-12.
Jalihal PC, Rajoriya V, Kashaw V. Design synthesis and evaluation of new derivative of 1,2,4-triazoles for antimicrobial and anti-inflammatory activity. Int J Curr Pharm Res. 2018 Jul;10(4):29-35. doi: 10.22159/ijcpr.2018v10i4.28455.
Thomas A, VB, SSKU, VMV. Development of novel 1, 3, 4-thiadiazoles as antitubercular agents: synthesis and in vitro screening. Int J Curr Pharm Res. 2023 May;15(3):37-41. doi: 10.22159/ijcpr.2023v15i3.3009.
Verma SK, Verma R, Verma S, Vaishnav Y, Tiwari SP, Rakesh KP. Anti-tuberculosis activity and its structure-activity relationship (SAR) studies of oxadiazole derivatives: a key review. Eur J Med Chem. 2021 Jan 1;209:112886. doi: 10.1016/j.ejmech.2020.112886, PMID 33032083.
Bakale RD, Sulakhe SM, Kasare SL, Sathe BP, Rathod SS, Choudhari PB. Design synthesis and antitubercular assessment of 1, 2, 3-triazole incorporated thiazolylcarboxylate derivatives. Bioorg Med Chem Lett. 2024 Jan 1;97:129551. doi: 10.1016/j.bmcl.2023.129551, PMID 37979730.