
Int J Chem Res, Vol 9, Issue 1, 1-6Research Article
SPECTROSCOPIC AND MOLECULAR DOCKING ANALYSIS OF PHYTOCONSTITUENT ISOLATED FROM SOLENOSTEMON MONOSTACHYUS AS POTENTIAL CYCLOOXYGENASE ENZYMES INHIBITOR
HARUNA BABA1 , SAMUEL J. BUNU2*
, SAMUEL J. BUNU2*
1Department of Pharmaceutical and Medicinal Chemistry, Faculty of Pharmacy, University of Calabar, Cross River State, Nigeria. 2Department of Pharmaceutical and Medicinal Chemistry, Faculty of Pharmacy, Niger Delta University, Wilberforce Island, Bayelsa State, Nigeria
*Corresponding author: Samuel J. Bunu; *Email: [email protected]
Received: 15 Oct 2024 Revised and Accepted: 28 Nov 2024
ABSTRACT
Objective: The study aimed to assess the anti-inflammatory activity of kaempferol-7-O-methylether, a phytoconstituent isolated from Solenostemon monostachyus.
Methods: Elemental characterization and structural elucidation of the dichloromethane fraction of the leaves using flash chromatography, gel filtration, thin layer chromatography, infrared spectroscopy, nuclear magnetic resonance, and mass spectrometry resulted in the isolation of the novel kaempferol-7-O-methylether.
Results: Based on the purported anti-inflammatory ethnobotanical qualities, we performed in silico molecular docking experiments to determine if kaempferol-7-O-methyl ether mediates the anti-inflammatory and analgesic activities, with acetylsalicylic acid, celecoxib, and diclofenac as standard controls. The molecular docking was performed using the crystal structures of the proteins PDB ID: 7M8W, 3N8Y, and 3LN1. The binding affinity of kaempferol-7-O-methyl ether to 7M8W, 3N8Y, and 3LN1 were-5.53,-7.38, and-7.22 kcal/mol, respectively. Kaempferol-7-O-methyl ether had a higher affinity than 15R-methyl-prostaglandin D2 and acetylsalicylic acid.
Conclusion: the novel Kaempferol-7-O-methyl ether isolated from S. monostachys leaves when modified, could serve as a potential lead molecule in the suppression of human target proteins or enzymes linked with pain and inflammation pathophysiology.
Keywords: Solenostemon monostachyus, Phytoconstituents, Anti-inflammatory, In silico
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/)
DOI: http://dx.doi.org/10.22159/ijcr.2025v9i1.241 Journal homepage: https://ijcr.info/index.php/journal
INTRODUCTION
Natural products are known to possess antimicrobial [1, 2], anti-bacterial [3], antiviral [4], aphrodisiac [5], antimalarial [6], anthelmintic [7], hypoglycemic activities [8-14]. Previous studies have compared the nutritional value of conventional medicines with edible medicinal plants [15]. There is a need for standardization, quality control, and post-marketing surveillance of natural products to safeguard the end users [16]. There are also several active synthetic drug molecules with good anti-inflammation properties [17-19]. Research has identified cinnamic acid and chalcone derivatives as possible lead compounds in the design and development of anti-inflammatory medicines [20, 21]. Medicinal plants are believed to be less toxic compared to synthetic agents [22]. The use of plant extracts, fractions, and molecules is a novel approach that offers outstanding opportunities for discovering appropriate medicinal products, leading to polypharmacology [23].
Solenostemon monostachys (P. Beauv.) Briq. (Lamiaceae) (fig. 1), is an herb native to West and Central Africa, a slightly succulent, aromatic plant that can grow up to 100 cm tall and is often found in rocky savannahs. The Ibibio of the Niger Delta region of Nigeria uses aerial parts as decoctions in the treatment of stomach ulcers, fever and malaria, hemorrhoids, inflammatory diseases, hypertension, and pains [24]. Studies have found that S. monostachyus extracts may have anti-inflammatory, analgesic, anti-plasmodial, and antipyretic properties [25]. These actions may be mediated by its chemical constituents, and the previously reported analgesic activity results suggest both central and peripheral pathways comparable to acetylsalicylic acid and diclofenac actions [24].

Fig. 1: Solenostemon monostachys (P. Beauv.) Briq. (Lamiaceae)
Our previous study isolated Kaempferol-7-O-methylether from S. monostachyus [26]. Inflammatory cells produce vasoactive amines and peptides, eicosanoids, proinflammatory cytokines, and acute-phase proteins, which control the inflammatory process by preventing further tissue damage and eventually leading to healing and tissue function restoration [27]. Given the observed anti-inflammatory and analgesic properties in mouse models, we undertook an in silico investigation of the chemical extracted to assess its potential to suppress the inflammatory and analgesic biological pathways.
MATERIALS AND METHODS
Design and setting of the study
The study design was a retrospective-perspective model, where reported or perceived ethnobotanical uses of S. monostachyus were utilized to access the phytochemical (kaempferol-7-O-methylether) isolated from the plant. The plant was obtained in Benin City (Nigeria) and validated by the Department of Pharmacognosy, Faculty of Pharmacy, University of Benin, under the herbarium number 1023. The plant leaves were selected, washed, dried, and ground into powder (500 g).
Structure elucidation
The powdered leaves were thoroughly extracted by cold maceration with dichloromethane (DCM), followed by methanol (MeOH). The DCM fraction was eluted gradient-wise using flash column chromatography using hexane and ethyl acetate. The resulting fractions were separated into five groups (A-E) based on their thin layer chromatography (TLC) profiles and concentrated at low temperatures. Subsequent gel filtration of fraction a on Sephadex LH-20 produced eluates with a single spot on the TLC [28]. The solvent was removed at 40 ℃, leaving behind yellow crystals that were characterized using infrared (IR) spectroscopy, 1H and 13C nuclear magnetic resonance (NMR), and mass spectrometry (MS) as previously reported [26].
In silico studies
Molecular docking of the binding protein, ketamine, and its analogs (ligands) was performed using the Schrodinger suit [29, 30]. The XFEL crystal structure of prostaglandin D2 receptor CRTH2 co-crystalized with 15R-methyl-prostaglandin D2 (PDB ID: 7M8W) [31], the structure of aspirin acetylated cyclooxygenase-1 in complexed with diclofenac (PDB ID: 3N8Y) [32], and the structure of celecoxib bound to the COX-2 active site (PDB ID: 3LN1) proteins were used in the in silico molecular docking investigations. As benchmarks, we employed acetylsalicylic acid, celecoxib, and diclofenac as controls, with our test compound, Kaempferol-7-O-methyl ether (3,5-dihydroxy-2-(4-hydroxyphenyl)-7-methoxy-4H-chromen-4-one).
RESULTS
The results obtained from the study are presented in tables and charts. Table 1 describes the physiochemical characteristics, spectroscopic, and elemental parameters of the isolated compound from S. monostachyus.
Table 1: Chemical characteristics
| Melting point: 225-227 °C | Crystals color/appearance: yellow | Obtained yield: 20 mg |
| Infrared (IR) spectroscopy [KBr] (Vmaxcm-1) | Mass spectrometry [EI-MS m/z (%)] | |
| -OH | 3480 | Sharp band |
| -OH | 3260 | broadband |
| C=O | 2463, 2364 1654, 1607 |
|
| 1H NMR (DMSO-d6) δ ppm | 13C-NMR (DMSO-d6) δ ppm | |
| 3H, s,-OCH3- | 3.9 | Observed peaks: 53.0, 94.4, 99.3, 103.3, 104.1, 116.4, 121.6, 128.9, 130.5, 137.4, 157.8, 161.6, 161.9, 164.2, 164.6, 182.2 |
| 1H, d, J = 2 Hz, C6-H | 6.2 | |
| 1H, d, J = 2 Hz, C8-, H | 6.5 | |
| 2H, d, J = 8.8 Hz, H-3', H-5' | 6.9 | |
| 2H, d, J = 8.8 Hz, H-2', H 6' | 7.9 | |
| 1H, s, OH | 10.3 | |
| 1H, s, OH | 10.8 | |
| 1H, s, OH | 12.9 |
Table 2 gives an overview of the protein-ligand binding affinity (docking scores) of the isolated compound Kaempferol-7-O-methylether obtained from the phytoconstituents of Solenostemon monostachys against three cyclooxygenase enzymes ((proteins) implicated in inflammatory pathophysiology.
Table 2: Molecular docking results
Molecule ID |
PDB ID: 7M8W | PDB ID: 3N8Y | PDB ID: 3LN1 |
| Docking score (kcal/mol) | Glide emodel | Docking score (kcal/mol) | |
| Kaempferol-7-O-methylether | -5.53 | -50.80 | -7.38 |
| Acetylsalicylic acid | -6.19 | -46.45 | -6.35 |
| Celecoxib | -6.12 | -54.60 | - |
| Diclofenac | -7.66 | -66.81 | -8.56 |
| 15R-methyl-prostaglandin D2 | -7.43 | -74.52 | -4.93 |
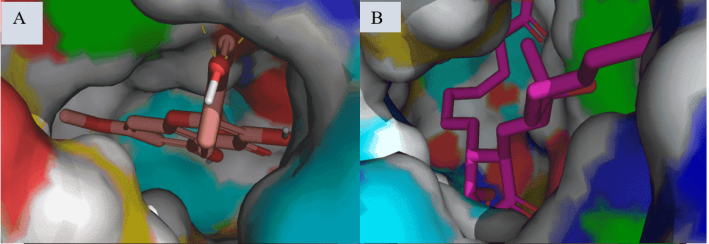
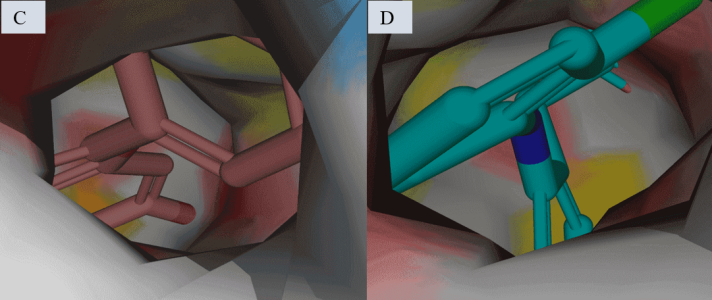
Fig. 2: Surface binding pose of Kaempferol-7-O-methylether (A) and 15R-methyl-prostaglandin-D2 (B) with 7M8W. Kaempferol-7-O-methylether (C) and Diclofenac (D) with 3N8Y (B). Kaempferol-7-O-methylether (E) and Celecoxib (F) with 3LN1
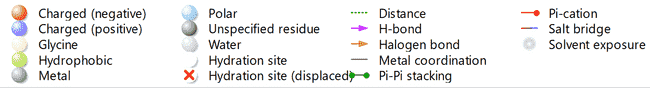
Fig. 3: 7M8W Protein-ligand interactions: 2D interactions of Kaempferol-7-O-methylether (A) and 15R-methyl-prostaglandin-D2 (B), Diclofenac (C), Acetylsalicylic acid (D), and Celecoxib (E) with 7M8W. 3D interactions of Kaempferol-7-O-methyl ether (F) and 15R-methyl-prostaglandin-D2 (G), Diclofenac (H), Acetylsalicylic acid (I), and Celecoxib (J) with 7M8W
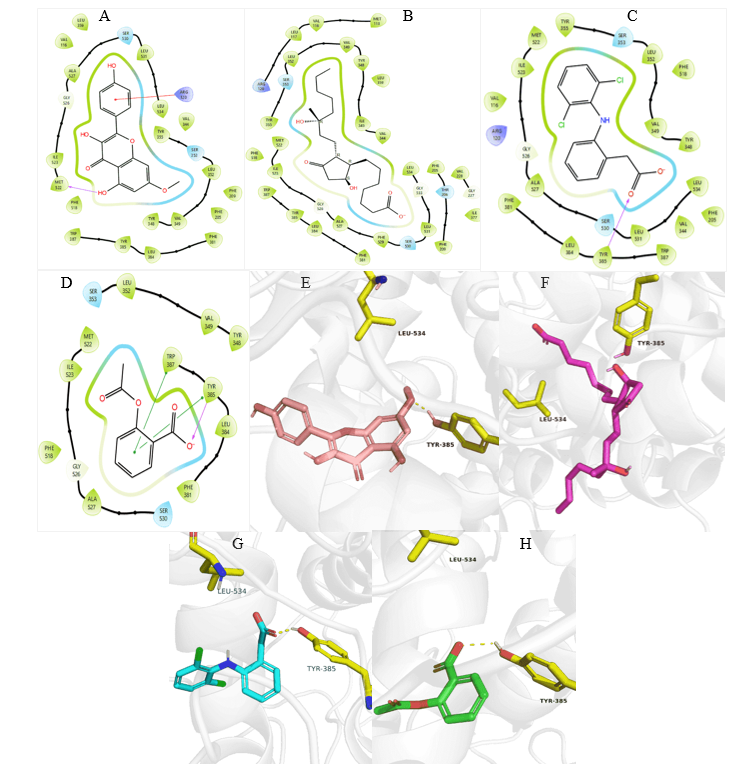
Fig. 4: 3N8Y Protein-ligand interactions: 2D interactions of Kaempferol-7-O-methylether (A) and 15R-methyl-prostaglandin-D2 (B), Diclofenac (C), and Acetylsalicylic acid (D) with 7M8W. 3D interactions of Kaempferol-7-O-methyl ether (E) and 15R-methyl-prostaglandin-D2 (F), Diclofenac (G), and Acetylsalicylic acid (H) with 3N8Y
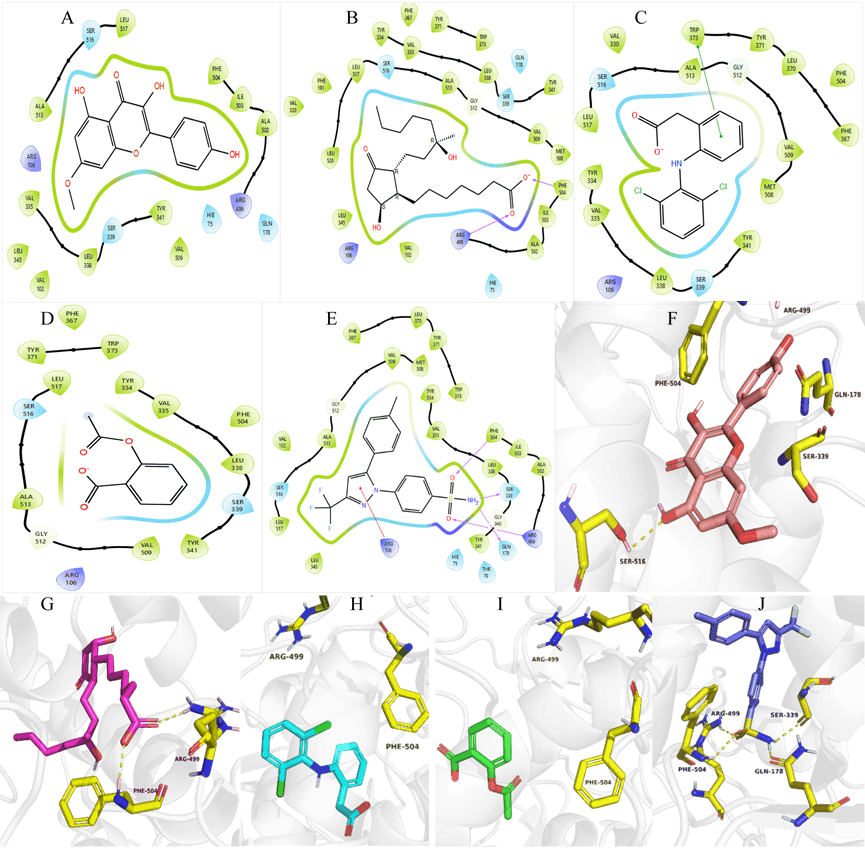
Fig. 5: 3LN1 Protein-ligand interactions: 2D interactions of Kaempferol-7-O-methylether (A) and 15R-methyl-prostaglandin-D2 (B), Diclofenac (C), Acetylsalicylic acid (D), and Celecoxib (E) with 7M8W. 3D interactions of Kaempferol-7-O-methylether (F) and 15R-methyl-prostaglandin-D2 (G), Diclofenac (H), Acetylsalicylic acid (I), and Celecoxib (J) with 3LN1
DISCUSSION
The molecule weighs 300 g, as demonstrated by the presence of a molecular ion peak at m/z 300 in the EI positive mass spectrum (table 1). The compound's infrared spectra (IR) revealed two absorption bands at 3480 and 3260 cm-1 for the chelated and non-chelated OH groups, respectively. The IR spectrum showed absorption bands at 1654 and 1607 cm-1, indicating the existence of α, β-unsaturated C=O, and at 1503, 1420 cm-1, suggesting stretching of the ether function (table 1). The 1HNMR spectra revealed two meta-coupled doublets and two ortho-coupled A2B2-type doublets in the aromatic region. These proton signals showed the presence of a tetra-and 1,4-disubstituted phenyl ring. The 13CNMR chemical shift of carbon signals at δ 130.5 identified the later ring as a p-hydroxyphenyl system26. The aliphatic part of the 1HNMR spectrum showed an integrated singlet for 3H at δ 3.9, attributed to the-OCH3 group at the C-7 position. This is supported by the downfield 13C-chemical shift of C-7 at δ 164.6. The 13CNMR spectra confirmed the presence of the C=O group at δ 182.2, benzylic carbon (C-2) at δ 157.8, and oxygen-bonded ethylenic carbon (C-3) at δ 137.4. This resulted in the first discovery and isolation of kaempferol-7-O-methylether [26].
Computer-aided drug design, particularly molecular docking, is an effective method for predicting possible enzymes or proteins as therapeutic targets for a variety of human disorders [33, 34]. The in silico assessment of the Kaempferol-7-O-methyl ether to predict possible binding target protein or enzymes (table 2) following reported ethnomedicinal properties of Solenostemon monostachys plant (fig. 2). The majority of reports indicated that the plant has been utilized in several cultures as an antipyretic, analgesic, or anti-inflammatory remedy. Hence, from the three unique biological target proteins used in the study, Kaempferol-7-O-methyl ether was observed to possess appreciable binding affinity to both proteins compared with the standards and co-crystalized inhibitors. Kaempferol-7-O-methyl ether interaction with 7M8W, showed a binding affinity of-5.53 kcal/mol (fig. 3). This affinity level was not as good as the standard molecules, including 15R-methyl-prostaglandin D2 (-7.43 kcal/mol), diclofenac (-7.66 kcal/mol), celecoxib (-6.12 kcal/mol), and acetylsalicylic acid (-6.19 kcal/mol) respectively.
Again, the binding affinity of Kaempferol-7-O-methyl ether interaction with 3N8Y, showed comparable but less affinity with the standard molecules (fig. 4). Diclofenac, which is the reported standard inhibitor of this target, showed the highest binding/inhibitory affinity at-8.56kcal/mol compared with the other molecules including Kaempferol-7-O-methyl ether (-7.38 kcal/mol), acetylsalicylic acid (-6.35 kcal/mol), and15R-methyl-prostaglandin D2 (-4.93 kcal/mol). The interactions were observed between celecoxib and 3N8Y, further validating the COX-2 selective inhibitory nature of this compound, as this target is for COX-1. Finally, we conducted another analysis of our novel compound with 3LN1 protein (oxidoreductase COX-2 enzyme) (fig. 5). The binding affinity obtained were-11.26,-7.73,-7.22,-6.51, and-6.09 kcal/mol in the order Celecoxib, Diclofenac, Kaempferol-7-O-methyl ether, 15R-methyl-prostaglandin D2, and Acetylsalicylic acid, respectively. This indicates that Kaempferol-7-O-methyl ether had better affinity compared with 15R-methyl-prostaglandin D2, and acetylsalicylic acid.
CONCLUSION
The novel Kaempferol-7-O-methyl ether, isolated from the leaves of Solenostemon monostachys when modified, could serve as a potential lead compound in the inhibition of human target proteins or enzymes associated with pain and inflammation pathophysiology. The compound showed comparable inhibitory properties or affinity with the standard conventional molecules used in the study.
ACKNOWLEDGEMENT
The authors acknowledge the contributions of Prof. Cyril O. Usifoh of the Department of Pharmaceutical Chemistry, Faculty of Pharmacy, University of Benin, Nigeria, and Niger Delta University, Wilberforce Island, Bayelsa State, Nigeria
FUNDING
Nil
AUTHORS CONTRIBUTIONS
All authors contributed to the completion of this work. The final manuscript was read and approved by all authors.
CONFLICT OF INTERESTS
The authors declare no conflict of interest among the author
REFERENCES
Bunu SJ, Oyeintonbara M, Azibanasamesa OD, Martins OO, Ogechukwu CL. Phytochemicals quantification, TLC, and antimicrobial assessment of leaves and fruit extracts of Lasimorpha senegalensis (Schott) araceae. J Chem Res Adv. 2022;3(2):14-20.
Bunu SJ, Alfred-Ugbenbo D, Anumo DF, Adugo M, Angela RI. Secondary metabolites and antimicrobial profile of the crude stem and rhizome extracts of Lasimorpha senegalensis (Schott) araceae. Open Access J Chem. 2023;6(1):9-15.
Bunu SJ, Alfred Ugbenbo D, Owaba AD, Okelekele B. Determination of phytochemicals and anti-bacterial properties evaluation of the leaves extracts of Psidium guajava (L) myrtaceae. EJPHARMA. 2023;3(3):13-6. doi: 10.24018/ejpharma.2023.3.3.67.
Nwankwo OL, Bunu SJ, Miediegha O, Iloh ES, Adione NM, Onwuzuligbo N. Usefulness of herbal medicines in the prevention and management of coronavirus disease-2019 (COVID-19) and its symptoms: a review. J Pharmacogn Phytochem. 2022;11(2):68-78. doi: 10.22271/phyto.2022.v11.i2a.14378.
Owaba AD, Arueniobebh F, Bunu SJ, Rafiu OR, Johnson EC, Etim IE. Spectroscopic analysis, aphrodisiac potential of Carapa procera stem bark extract in male wistar rats and in silico studies of hexadecanoic and oleic acids on phosphodiesterase-5 and adenyl cyclase enzymes. Biomed J Sci Tech Res. 2024;54(5):46343-56.
Ogechukwu LN, Bunu SJ, Iloh ES. Standardization and antimalarial evaluation of the methanol and endophytic fungi extract isolated from the Azadirachta indica (Meliaceae) mice model. Niger J Clin Pharm Ther. 2021;1(1):87-98.
Chukwuemerie OL, Bunu SJ, Iloh ES, Ogujiuba CU, Obasi J, Onwuzuluigbo CC. Evaluation of the anthelmintic property of the endophytic extract of <em>Senna alata</em> in mice model. J Med Plants Stud. 2022;10(3):52-4. doi: 10.22271/plants.2022.v10.i3a.1425.
Nwankwo OL, Bunu SJ, Aziakpono OM. Hypoglycemic activity of endophytic extract of Senna alata in STZ-induced diabetic mice model. J Integr Health Sci. 2021;9(2):75-80. doi: 10.4103/jihs.jihs_25_21.
Nwankwo OL, Chukwuebuka OC, Collins OO, Samuel BJ, Obasi JC, Iloh ES. Quantitative phytochemical analysis of the fungus endophytic extracts isolated from Azadirachta indica using gas chromatography- flame ionization detector. J Drug Deliv Ther. 2021;11(5):80-3. doi: 10.22270/jddt.v11i5.4999.
Bunu SJ, Alfred Ugbenbo D, Kogoro AC, Adesegun KJ, Vaikosen EN, Chukwuemerie OL. Quantification of secondary metabolites and chromatographic analysis of Allium cepa, Liliaceae ethanolic extract. IOSR JPBS (IOSR-JPBS). 2023;18(2):16-22.
Chukwuemerie OL, Bunu SJ, Iloh ES, Onwuzuluigbo CC, Onyegbule FA, Okoye FB. Antimicrobial properties and characterization of secondary metabolites obtained from Curvularia lunata, an endophyte of Azadirachta indica. J Drug Deliv Ther. 2022;12(6):110-9. doi: 10.22270/jddt.v12i6.5676.
Bunu SJ, Okei JO, Miediegha O, Ebeshi BU, Chukwuemerie OL. Assessment of secondary metabolites and thin-layer chromatographic analysis of Carica papaya (Caricaceae) leaves ethanolic extract. J Pharm Res Int. 2023;35(36):21-8. doi: 10.9734/jpri/2023/v35i367489.
Owaba AD, Miediegha O, Bunu SJ. Comparative physicochemical, spectroscopic and elemental analyses of the seed oils of Parinari excelsa Sabinus and Chrysobalanus icaco linn. chrysobalanacea. AJPHS. 2022;12(1):2615-23. doi: 10.5530/ajphs.2022.12.02.
Owaba AD, Bunu SJ, Javier TO. Physicochemical and elemental assessment of vegetable oils in Yenagoa Metropolis, Bayelsa State, Nigeria. Eur J Pharm Med Res. 2021;8(10):10-4.
Ebeshi UB, Bunu JS, Vaikosen NE, Kashimawo JA, Enoch MU, Chukwuemerie LO. Quality assessment of ascorbic acid tablet formulations and comparative analysis with edible fruits. J Basic Soc Pharm Res. 2022;2(4):57-66. doi: 10.52968/27455330.
Miediegha O, Bunu SJ. Pharmacovigilance framework and extent of medications adverse reaction surveillance in Southern Nigeria. World J Pharm Res. 2020;9(6):2009-17.
Jacob BB, Baba H, Oluwadiya JO. Synthesis, Characterization and evaluation of anti-inflammatory and antimicrobial properties of some cinnamic acid derivatives. Niger J Pharm Res. 2020;16(1):1-8. doi: 10.4314/njpr.v16i1.1.
Awala EV, Bunu SJ, Haruna B, Oluwadiya JO. Synthesis, antimicrobial, and anti-inflammatory evaluation of epoxide and 4-methoxy-and 4,6-diphenyl-2-thiopyrimidine derivatives of chalcones. Scholars Acad J Pharm. 2019;8(8):436-42.
Ebiere Dode, Samuel Jacob Bunu, Onyinpere Rita Garando. Assessment of different brands of diclofenac tablets: an evaluation utilizing uv spectroscopy and disintegration test methods. World J Bio Pharm Health Sci. 2023;14(2):1-6. doi: 10.30574/wjbphs.2023.14.2.0201.
Bunu SJ, Miediegha O, Awala EV, Alfred Ugbenbo D, Baba H, Usifoh CO. Synthesis and in silico analysis of chalcone derivatives as potential prostaglandin synthetase inhibitors. Biomed J Sci Tech Res. 2024;55(2):46709-20.
Bunu SJ, Alfred Ugbenbo D, Miediegha O, Baba H. Characterization and molecular docking of cinnamic acid derivatives: potential inhibitors of cyclo-oxygenase enzymes. Innovare J Life Sci. 2023;2023(11):41-6.
Karimi A, Majlesi M, Rafieian Kopaei M. Herbal versus synthetic drugs; beliefs and facts. J Nephropharmacol. 2015;4(1):27-30. PMID 28197471.
Nasim N, Sandeep IS, Mohanty S. Plant-derived natural products for drug discovery: current approaches and prospects. Nucleus (Calcutta). 2022;65(3):399-411. doi: 10.1007/s13237-022-00405-3, PMID 36276225.
Okokon JF, Davis K, Nwidu LL. Anti-inflammatory and antinociceptive activities of Solenostemon monostachyus aerial part extract in mice. Avicenna J Phytomed. 2016;6(3):284-94. PMID 27462551.
Okokon J, Davis KA, Azare BA. Antipyretic and antimalarial activities of Solenostemon monostachyus. Pharm Biol. 2016;54(4):648-53. doi: 10.3109/13880209.2015.1070880, PMID 26474350.
Baba H, Usifoh CO. Isolation and characterization kaempferol-7-O-methyl ether from Solenostemon monostachyus (P. Beauv.) Briq. (Lamiaceae). Niger J Pharm Appl Sci Res. 2014;3(1):32-7.
Abdulkhaleq LA, Assi MA, Abdullah R, Zamri Saad M, Taufiq Yap YH, Hezmee MN. The crucial roles of inflammatory mediators in inflammation: a review. Vet World. 2018;11(5):627-35. doi: 10.14202/vetworld.2018.627-635, PMID 29915501.
Bunu SJ, Vaikosen NE, Nnadozie WK. Chloroquine phosphate metabolism and gender-based phenotypic analysis in healthy subjects’ urine following oral administration. Pharm Biomed Res. 2020;6i(s1):37-44. doi: 10.18502/pbr.
Maestro. Maestro programme. LLC. Portland: Schrödinger; 2020.
Seeliger D, de Groot BL. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J Comput Aid Mol Des. 2010;24(5):417-22. doi: 10.1007/s10822-010-9352-6, PMID 20401516.
Liu H, Deepak RN, Shiriaeva A, Gati C, Batyuk A, Hu H. Molecular basis for lipid recognition by the prostaglandin D2 receptor CRTH2. Proc Natl Acad Sci USA. 2021;118(32):e2102813118. doi: 10.1073/pnas.2102813118, PMID 34341104.
Sidhu RS, Lee JY, Yuan C, Smith WL. Comparison of cyclooxygenase-1 crystal structures: cross-talk between monomers comprising cyclooxygenase-1 homodimers. Biochemistry. 2010;49(33):7069-79. doi: 10.1021/bi1003298, PMID 20669977.
Jacob Bunu S, Cai H, Wu L, Zhang H, Zhou Z, Xu Z. TRIP13-a potential drug target in cancer pharmacotherapy. Bioorg Chem. 2024;151:107650. doi: 10.1016/j.bioorg.2024.107650, PMID 39042962.
Li X, Zhang H, Dong S, Gao X, Sun H, Zhou Z. Design, synthesis, and biological evaluation of novel 1-amido-2-one-4-thio-deoxypyranose as potential antitumor agents for multiple myeloma. Bioorg Med Chem. 2024;111:117843. doi: 10.1016/j.bmc.2024.117843, PMID 39083980.