
Int J Chem Res, Vol 8, Issue 1, 1-5Review Article
REVIEW OF THERMODYNAMIC AND PHYSICO-CHEMICAL PARAMETERS INFLUENCING METHYLENE BLUE ADSORPTION ON ACTIVATED CHARCOAL OF NATURAL ORIGIN
MAMADOU BALDE1*, ADAMA DIEDHIOU1, IDRISSA NDOYE1, HAROUNA TIRERA1, ROKHAYA SYLLA GUEYE1, YORO TINE1, KHADIDIATOU THIAM2, NANGO GUEYE1, MATAR SECK1, DJIBRIL FALL1, ALASSANE WELE1
1Laboratory of Physical Chemistry, Mineral Chemistry, Organic Chemistry and Therapeutic Chemistry, Faculty of Medicine, Pharmacy and Odontology, Cheikh Anta Diop University (UCAD), BP-5005, Dakar, Senegal. 2Laboratory of Analytical Chemistry and Bromatology, Faculty of Medicine, Pharmacy and Odontology, Cheikh Anta Diop University (UCAD), BP-5005, Dakar, Senegal
*Corresponding author: Mamadou Balde; *Email: [email protected]
Received: 10 Jan 2024 Revised and Accepted: 25 Feb 2024
ABSTRACT
Methylene blue (MB) is a phenothiazine derivative used in microbiology, surgery, diagnostics and as a sensitizer in the photo-oxidation of organic pollutants. These numerous uses of methylene blue could lead to its accumulation in wastewater, causing harmful effects on the environment and living beings. To combat these harmful effects, numerous wastewater treatment processes, particularly physicochemical, were implemented. Adsorption techniques are particularly used to find new natural, biodegradable and inexpensive adsorbents to treat colored waste from structures such as medical and pharmaceutical laboratories. The aim of the present study was to carry out a relevant bibliographical review of the physico-chemical and thermodynamic parameters that can influence this adsorption of methylene blue on activated carbons of natural origin. The results showed a remarkable elimination of methylene blue. However, parameters such as particle size, adsorbent mass, pH, contact time, initial methylene blue concentration, agitation speed and temperature showed that the adsorption capacity of MB on biosorbents was influenced by these quantities. Similarly, the positive and negative enthalpy values (ΔH°) indicated that adsorption process could be endothermic or exothermic. Other thermodynamic parameters, such as the negative value of Gibbs free energy (ΔG°) and the positive value of entropy (ΔS°), also showed that the adsorption process was feasible and spontaneous.
Keywords: Adsorption, Methylene blue, Bisorbents, Thermodynamic, Physico-chemical
© 2024 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/)
DOI: http://dx.doi.org/10.22159/ijcr.2024v8i2.229 Journal homepage: https://ijcr.info/index.php/journal
INTRODUCTION
Dyes are water-soluble, dispersible, aromatic, synthetic organic compounds with potential applications in industry. From an environmental perspective, the disposal of synthetic dyes is a major concern because some dyes and their breakdown products are carcinogenic and toxic [1, 2]. Furthermore, the discharge of colored effluents into the environment is not only unpleasant but also represents a real danger for humans and their environment, due to their stability and their low biodegradability [3]. Concentrations of these toxic pollutants in wastewater far exceed limits set by the World Health Organization (WHO) and the Environmental Protection Agency (EPA) in many countries [4]. The expansion of industrial activities (pharmaceuticals, cosmetics, textiles, plastics, etc.) is often accompanied by environmental pollution. Pollutants generated by these activities are generally released into the environment without prior treatment and are likely to reach groundwater and pose public health problems. Among these pollutants, is methylene blue, used in wood and paper dyeing and in the pharmaceutical industry. However, methylene blue can cause permanent breathing difficulties, nausea, vomiting, gastritis, mental confusion, tissue necrosis, etc. [5]. To prevent the harmful effects of methylene blue, several physicochemical and biological methods have been developed to remove these dyes, including adsorption, nanofiltration, electrochemical oxidation, precipitation, ozonation, electrocoagulation etc. [6, 7]. Thus, several methods have been used to reduce the harmful effects of effluent discharges, such as biological methods, but the results obtained have often been unsatisfactory due to the composition and nature of these discharges, which were difficult to biodegrade [8, 9]. Indeed, some of these methods had revealed their limits, linked on the one hand to the production of toxic by-products and on the other hand, to the sometimes very high financial cost of their implementation. To overcome these difficulties, research is increasingly moving towards less expensive, flexible and efficient adsorption methods [10, 11]. These alternative methods use a variety of solid materials (banana peel, orange peel, palm waste, sunflower stalks, eucalyptus leaves, date nuts, lemongrass ash, pineapple peel, Parmotrema dilatatum lichen, Euphorbia tirucalli L wood, Peruvian wood, etc.) with the aim of producing activated charcoal to remove soluble and insoluble pollutants, without generating hazardous by-products [12-18].
MATERIELS AND METHODS
Methylene blue (MB), or tetramethylthionine chloride (fig. 1), is a cationic dye and one of the most widely used pigments in the dyeing industry [19]. It is solid in the form of odorless crystals, soluble in water and slightly less so in ethyl alcohol. It is also used in microbiology, in surgery, in diagnosis and as a sensitizer in the photo-oxidation of organic pollutants, its action residing essentially in its redox properties. The transition from its oxidized form to its reduced form, or inversely, causes a change in color, which in turn gives an indication of the nature of the environment in which it is found (fig. 2) [20].
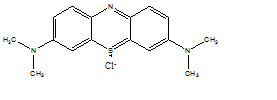
Fig. 1: Structure of methylene blue
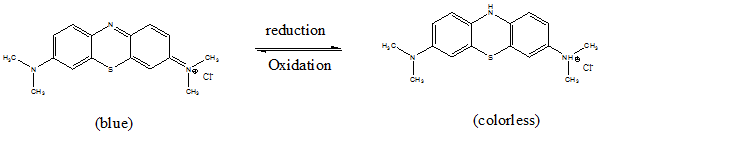
Fig. 2: Redox reaction of methylene blue
The publications used were obtained by consulting several databases, including Elsevier, Web of Science, PubMed, Scopus, Google Scholar, etc.
DISCUSSION
Effect of particle size
The particle size of biosorbent can affect the adsorption capacity. Indeed, the results showed that the amount of methylene blue adsorbed increased with decreasing particle size [21–23]. This could be explained by the fact that the finer the particle size, the greater the specific surface area and, therefore, the higher the adsorption percentage.
Effect of contact time
The contact time corresponds to the adsorption/desorption equilibrium, or equilibrium state in which the substrate saturates the support. Analysis of the results showed that the amounts of adsorbed dyes increased with contact time [24–26], then subsequently, the curves described a plateau, reflecting the biosorption chemical equilibrium. This balance could be explained by the occupation of the majority of sites by coloring molecules. The results also showed that the elimination rate increased rapidly, reaching 50% in the first minutes of the experiment, after which the increase was slow until equilibrium was reached. The increase in removal rate in the first part might be due to the availability of more vacant adsorption sites on the adsorbent surface at the initial stage of adsorption. Additionally, the MB molecules were relatively medium in size and could be easily diffused into the internal pores of the adsorbent until saturation, which would reduce the mass transfer between the liquid and solid phases over time. This would result in a reduction in the adsorption rate, and a plateau corresponding to the stationary state would be observed, that is to say a saturation of the sites available on the adsorbent.
Effect of initial dye concentration
The study of the effects of MB concentration on adsorption capacity was carried out with known initial MB concentrations while keeping all other conditions constant. The reported data showed that the increase in initial MB concentration was proportional to the adsorption capacity of the biosorbent [27, 28]. These results could be explained by the accelerated diffusion of MB ions on the adsorbent due to the increased driving force of the concentration gradient between the dye ions and the adsorbent. This would lead to increased mass transfer of MB ions onto the adsorbent surface. The increase in adsorption capacity could be attributed to the ratio of available active adsorption sites to the initial number of MB molecules. These results were in agreement with those observed in other works [29, 30]. However, at higher MB concentrations, lower adsorption efficiencies were observed due to saturation of the adsorption sites [31, 32].
Effect of adsorbent dose
The dose of biosorbent is also one of the parameters that can have a major impact on the adsorption process. Indeed, results reported in various studies showed that the removal rate gradually increased with increasing biosorbent dose while the adsorption capacity decreased [33, 34]. The increase in MB removal rate could be attributed to greater availability of adsorption active sites and functional groups on the biosorbent surface at the beginning of the experiment. However, the continuous increase in the adsorbent dose could represent a limit to the adsorption phenomenon due to the possible formation of an agglomerate which would disrupt the binding of the MB.
Effect of stirring speed
The reported results showed an increase in the amount of biosorbed MB as a function of shaking speed at relatively moderate values. However, at very high stirring speeds, a decrease in dye adsorption was observed [35]. This could be explained by the fact that an increase in the stirring speed would make the suspension inhomogeneous and, therefore, the binding of the dye ions to the surface of the biosorbent would be disrupted.
Effect of pH
pH is an important factor in studying the adsorption process because it could influence both the structure of the adsorbent and adsorbate, as well as the adsorption mechanism. Indeed, pH could affect both the surface charge of the material as well as the distribution and speciation of cations [36–38]. For this reason, several studies have reported the effect of pH on MB adsorption rates over a wide range of values from pH = 1 to pH = 12 [39]. In these studies, the pH of the solution was adjusted to the desired values by adding solutions (0.1 N) of hydrochloric acid (HCl), nitric acid (HNO₃) or sodium hydroxide (NaOH). The mixtures were stirred at constant speed for homogenization for one hour then filtered. Then, to elucidate the role of the net charge carried by the adsorbent surface in the binding of dye ions, the pH of zero charge point (pHPZC) was determined. This point corresponds to the pH value of the medium for which the resultant of the positive and negative charges on the surface is zero. In the example of the study on the adsorbent obtained from the deactivated Parmotrema dilatatum lichen, the pHPZC was 6.5. Consequently, in a strongly acidic pH environment, the surface of the deactivated lichen is charged with positive ions (H+), making it more difficult to adsorb the positively charged MB cationic dye in aqueous solution. As pH approaches the pHPZC value, the number of positively charged sites decreases while the number of negatively charged sites increases. This favors MB adsorption by electrostatic attraction between hydroxide ions (OH-) and cationic dye ions. However, an opposite trend was obtained for a strongly acidic pH medium in the case of an anionic dye such as orange II. Indeed, the surface of the deactivated lichen is charged with positive ions (H+), which promotes the adsorption of the anionic dye by electrostatic attraction between the cationic and anionic ions [40, 41]. In most studies, in the pH range above 5, it was noted that the adsorbed amount could exceed 50% [42, 43]. This could be explained by the fact that at low pH (acidic environment), the surface of the adsorbent would be surrounded by H+ions, thus reducing the interaction of MB ions (cationic dye) with the adsorbent sites due to electrostatic repulsion and competition between H+ions and cationic dye for adsorption sites. On the other hand, at higher pH values (basic medium), the concentration of H+ ions decrease, resulting in good interaction between the dye ions and the adsorbent surface sites. A similar observation was reported for the adsorption of MB onto wheat bran [44]. In the case of anionic dyes, as the pH increases, the number of negatively charged sites increases and there is competition between the negatively charged hydroxide ions and the anionic dye for sorption sites, and the adsorption rate decreases [45].
Effects of temperature and thermodynamic parameters
Temperature is also a factor that can affect the adsorption process. Thus, in order to determine the optimal temperature to obtain optimal adsorption capacity, several studies were carried out at different temperatures ranging from 0 to 60 °C. According to the reported results, the adsorption capacity of MB increased with temperature, reaching a maximum at a temperature of 25 °C. However, above this temperature value, a gradual decrease in dye removal efficiency was observed, revealing that the adsorption of MB onto the adsorbent was exothermic in nature. Thus, overheating would negatively affect the adsorption process in accordance with Le Chatelier's principle, which could lead to an increase in desorption kinetics [46, 47]. Thus, according to these results, increasing temperature is detrimental to the adsorption phenomenon and consequently, the best results were obtained in the room temperature range. However, other results also revealed that the amount of dye adsorbed was proportional to the increase in temperature, indicating that the adsorption phenomenon could also be endothermic in nature [48]. This increase in adsorption with increasing temperature could be explained by adsorbent-adsorbate chemical interactions leading to the creation of new adsorption sites depending on the matrices studied. This effect of temperature on MB adsorption capacity is consistent with the results obtained by Khelifi et al. [49]. In addition, thermodynamic parameters such as enthalpy variation (ΔH°), entropy variation (ΔS°) and Gibbs free energy (ΔG°) were determined in the various works to assess the feasibility and nature of the adsorption process. Indeed, a negative value of ΔH° indicates an exothermic process and a positive value indicates an endothermic process. On the other hand, the parameters ΔS° and ΔG° were used to identify the feasibility and spontaneity of the sorption process. The Gibbs free energy (ΔG°) of the adsorption reaction was determined in these studies from the following equation:
ΔG° =-RTlnKc
Where Kc is the thermodynamic equilibrium constant, T is the temperature, R is the perfect gas constant (8,32 J mol-1K-1).
Similarly, the relationship between ΔG°, ΔH° and ΔS° was expressed by the following equations:
ΔG° = ΔH°-TΔS°
lnKc =-ΔG°/RT =-ΔH°/RT+ΔS°/R
The results reported in these studies showed a negative value for the enthalpy variation (ΔH°, kJ mol-1), confirming the exothermic nature of MB ion adsorption to the adsorbent surface. In addition, the negative value of the Gibbs free energy (ΔG°, en kJ mol-1) and the positive value of the entropy (ΔS°, J mol-1 K-1) revealed that the adsorption process was feasible and spontaneous [50, 51]. Similar results were obtained by Srivastava and al. [52]. The evolution of the ΔG° value towards increasingly negative values with increasing temperature confirmed that MB adsorption was favored with increasing temperature [53]. Other works have also reported a positive value of ΔH° during the methylene blue adsorption process, indicating the endothermic nature of the process. This same trend towards the endothermic nature of the methylene blue adsorption process has also been reported in other works [54, 55].
CONCLUSION
According to the results reported in these various works, physicochemical and thermodynamic parameters had a strong influence on the adsorption process of methylene blue by biosorbents. Consequently, their values should be monitored when studying the adsorption process in order to obtain the best results.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
Research and drafting of the article were carried out by MB. The manuscript was reviewed by RSG, AD, ID, HT, YT, KT, NG, DF and MS. Supervision was provided by AW.
CONFLICT OF INTERESTS
The authors declare that they have no conflict of interest.
REFERENCES
Sakr F, Sennaoui A, Elouardi M, Tamimi M, Assabbane A. Etude de l’adsorption du bleu de methylene sur un biomateriau a base de cactus [Adsorption study of methylene blue on biomaterial using cactus]. J Mater Environ Sci. 2015;6(2):397-406.
Forgacs E, Cserhati T, Oros G. Removal of synthetic dyes from wastewaters: a review. Environ Int. 2004;30(7):953-71. doi: 10.1016/j.envint.2004.02.001, PMID 15196844.
Rosales E, Meijide J, Tavares T, Pazos M, Sanroman MA. Grapefruit peelings as a promising biosorbent for the removal of leather dyes and hexavalent chromium. Process Saf Environ Prot. 2016;101(1):61-71. doi: 10.1016/j.psep.2016.03.006.
Karri RR, Ravindran G, Dehghani MH. Wastewater sources, toxicity, and their consequences to human health. Soft. Comp Tech Sol Wast Wastewat Manag. 2021;1(1):3-33. doi: 10.1016/B978-0-12-824463-0.00001-X.
Mahmoud DK, Salleh MAM, Karim WAWA, Idris A, Abidin ZZ. Batch adsorption of basic dye using acid treated kenaf fibre char: Equilibrium, kinetic and thermodynamic studies. Chem Eng J. 2012;181-182:449-57. doi: 10.1016/j.cej.2011.11.116.
Belaid KD, Kacha S. Etude cinetique et thermodynamique de l’adsorption d’un colorant basique sur la sciure de bois. Rseau. 2011;24(2):131-44. doi: 10.7202/1006107ar.
Shukla A, Zhang YH, Dubey P, Margrave JL, Shukla SS. The role of sawdust in the removal of unwanted materials from water. J Hazard Mater. 2002;95(1-2):137-52. doi: 10.1016/S0304-3894(02)00089-4, PMID 12409244.
Feng Y, Yang F, Wang Y, Ma L, Wu Y, Kerr PG. Basic dye adsorption onto an agro-based waste material–sesame hull (Sesamum indicum L.). Bioresour Technol. 2011;102(22):10280-5. doi: 10.1016/j.biortech.2011.08.090, PMID 21962534.
Liu Y, Wang J, Zheng Y, Wang A. Adsorption of methylene blue by kapok fiber treated by sodium chlorite optimized with response surface methodology. Chem Eng J. 2012;184(1):248-55. doi: 10.1016/j.cej.2012.01.049.
Kannan N, Meenakshisundaram M. Adsorption of congo red on various activated carbons. A comparative study. Water Air Soil Pollut. 2002;138(1):289-305. doi: 10.1023/A.
Rafatullah M, Sulaiman O, Hashim R, Ahmad A. Adsorption of methylene blue on low-cost adsorbents: a review. J Hazard Mater. 2010;177(1-3):70-80. doi: 10.1016/j.jhazmat.2009.12.047, PMID 20044207.
Baseri JR, Palanisamy PN, Sivakumar P. Adsorption of reactive dye by a novel activated carbon prepared from thevetia peruviana. Int J Chem Res. 2012;3(2):36-41.
Liu J, Wang Z, Li H, Hu C, Raymer P, Huang Q. Effect of solid-state fermentation of peanut shell on its dye adsorption performance. Bioresour Technol. 2018;249(1):307-14. doi: 10.1016/j.biortech.2017.10.010, PMID 29054060.
Ofomaja AE. Kinetics and mechanism of methylene blue sorption onto palm kernel fibre. Process Biochem. 2007;42(1):16-24. doi: 10.1016/j.procbio.2006.07.005.
Ali A, Saeed K. Phenol removal from aqueous medium using chemically modified banana peels as low-cost adsorbent. Desalination and Water Treatment. 2016;57(24):11242-54. doi: 10.1080/19443994.2015.1041057.
Munagapati VS, Yarramuthi V, Kim Y, Lee KM, Kim DS. Removal of anionic dyes (reactive Black 5 and Congo red) from aqueous solutions using banana peel powder as an adsorbent. Ecotoxicol Environ Saf. 2018;148(1):601-7. doi: 10.1016/j.ecoenv.2017.10.075, PMID 29127823.
Hazourli S, Ziati M. Valorisation d’un residu naturel ligno-cellulosique en charbon actif-exemple des noyaux de dattes. Rev Energ Ren. 2007;1(1):187-92. https://www.researchgate.net/publication/228557063.
Fegousse A, Miyah Y, Elmountassir R, Lahrichi A. Valorization of pineapple bark for removal of a cationic dye sush as methylene blue. J Mater Environ Sci. 2018;9(8):2449-57.
Singh H, Chauhan G, Jain AK, Sharma SK. Adsorptive potential of agricultural wastes for removal of dyes from aqueous solutions. Journal of Environmental Chemical Engineering. 2017;5(1):122-35. doi: 10.1016/j.jece.2016.11.030.
Udrea ML, Ion RM. Modelling of methylene blue dye adsorption on beech and fir wood sawdust as adsorbent support materials. J Sci Arts. 2019;3(48):675-86.
Abdallah M, Hijazi A, Hamieh M, Alameh M, Rammal H. Etude de l’adsorption du Bleu de Methylene sur un biomateriau a base de l’eucalyptus selon la taille des particules treatment of industrial wastewater using a natural and biodegradable adsorbent based on Eucalyptus. J Mater Environ Sci. 2016;7(11):4036-48.
Kumar S, Gunasekar V, Ponnusami V. Removal of methylene blue from aqueous effluent using fixed bed of groundnut shell powder. J Chem. 2013;2013:1-5. doi: 10.1155/2013/259819.
Ozer A, Dursun G. Removal of methylene blue from aqueous solution by dehydrated wheat bran carbon. J Hazard Mater. 2007;146(1-2):262-9. doi: 10.1016/j.jhazmat.2006.12.016, PMID 17204366.
Agalya A, Palanisamy PN, Sivakumar P. Kinetics, equilibrium studies on removal of ionic dyes using a novel non-conventional activated carbon. Int J Chem Res. 2012;3(1):62-8.
Singh H, Dawa TB. Removal of methylene blue using lemon grass ash as an adsorbent. Carbon Lett. 2014;15(2):105-12. doi: 10.5714/CL.2014.15.2.105.
Slimani R, Anouzla A, Abrouki Y, Ramli Y, Antri SE, Mamouni R. Removal of a cationic dye-methylene blue-from aqueous media by the use of animal bone meal as a new low-cost adsorbent. J Mater Environ Sci. 2011;2(1):77-87.
Dobi Brice KK, Lynda E, Zoungranan Y, Tchirioua E. Use of deactivated lichens for the adsorption of two toxic dyes: crystal violet and methyl red. Asian J Sci Technol. 2020;11(4):10911-9.
Lansari F, Edjekouane M, Khelifi O, Boukheteche I, Laksaci I. Elimination of methylene blue by low-cost biomaterial prepared from local natural residue. AJRESD. 2020;2(1):60-6. doi: 10.46657/ajresd.2020.2.1.9.
Kousha M, Daneshvar E, Sohrabi MS, Jokar M, Bhatnagar A. Adsorption of acid orange II dye by raw and chemically modified brown macroalga stoechospermum marginatum. Chem Eng J. 2012;192(1):67-76. doi: 10.1016/j.cej.2012.03.057.
Rida K, Chaibeddra K, Cheraitia K. Adsorption of cationic dye methyl green from aqueous solution onto activated carbon prepared from brachychiton populneus fruit shell. Indian J Chem Technol. 2020;27(1):51-9. doi: 10.56042/ijct.v27i1.22949.
Abdelwahab O. Evaluation of the use of loofa-activated carbons as potential adsorbents for aqueous solutions containing dye. Desalination. 2008;222(1-3):357-67. doi: 10.1016/j.desal.2007.01.146.
Santhy K, Selvapathy P. Removal of reactive dyes from wastewater by adsorption on coir pith activated carbon. Bioresour Technol. 2006;97(11):1329-36. doi: 10.1016/j.biortech.2005.05.016, PMID 16040240.
Selengil U, Yıldız D. Investigation of the methylene blue adsorption onto waste perlite. DWT. 2022;262(1):235-47. doi: 10.5004/dwt.2022.28530.
Low LW, Teng TT, Alkarkhi AFM, Ahmad A, Morad N. Optimization of the adsorption conditions for the decolorization and COD reduction of methylene blue aqueous solution using low-cost adsorbent. Water Air Soil Pollut. 2011;214(1-4):185-95. doi: 10.1007/s11270-010-0414-0.
Muinde VM, Onyari JM, Wamalwa B, Wabomba J, Nthumbi RM. Adsorption of malachite green from aqueous solutions onto rice husks: kinetic and equilibrium studies. J Environ Prot. 2017;8(3):215-30. doi: 10.4236/jep.2017.83017.
Iorhuna BT, Wuana RA, Yiase SG, Awuhe TT, Isaac E. Effects of pH, ionic strength and temperature on the rate of oxidation of arsenic (iii) by dissolved organic matter, dom obtained from sawdust, groundnut husk, and rice husk. Int J Chem Res. 2022;6(1):33-9. doi: 10.22159/ijcr.2022v6i1.202.
Pathania D, Sharma S, Singh P. Removal of methylene blue by adsorption onto activated carbon developed from Ficus carica Bast. Arab J Chem. 2017;10(1):S1445-51. doi: 10.1016/j.arabjc.2013.04.021.
Wang XS, Zhou Y, Jiang Y, Sun C. The removal of basic dyes from aqueous solutions using agricultural by-products. J Hazard Mater. 2008;157(2-3):374-85. doi: 10.1016/j.jhazmat.2008.01.004, PMID 18262725.
Kifuani KM, Mayeko AKK, Vesituluta PN, Lopaka BI, Bakambo GE, Mavinga BM. Adsorption d’un colorant basique, Bleu de Methylene. En: Solution aqueuse, Sur un B. Issu de Dechets agricoles de Cucumeropsis Mannii Naudin. IJBCS. 2018;12(1):558-75. doi: 10.4314/ijbcs.v12i1.43.
Hor KY, Chee JMC, Chong MN, Jin B, Saint C, Poh PE. Evaluation of physicochemical methods in enhancing the adsorption performance of natural zeolite as a low-cost adsorbent of methylene blue dye from wastewater. J Clean Prod. 2016;118(1):197-209. doi: 10.1016/j.jclepro.2016.01.056.
Miyah Y, Lahrichi A, Idrissi M, Khalil A, Zerrouq F. Adsorption of methylene blue dye from aqueous solutions onto walnut shells powder: equilibrium and kinetic studies. Surf Interfaces. 2018;11(1):74-81. doi: 10.1016/j.surfin.2018.03.006.
Sakr F, Alahiane S, Sennaoui A, Dinne M, Bakas I, Assabbane A. Removal of cationic dye (methylene blue) from aqueous solution by adsorption on two type of biomaterial of South Morocco. Mater Today Proc. 2020;22(1):93-6. doi: 10.1016/j.matpr.2019.08.101.
Malik PK. Use of activated carbons prepared from sawdust and Rice-Husk for adsorption of acid dyes: a case study of acid yellow 36. Dyes Pigments. 2003;56(3):239-49. doi: 10.1016/S0143-7208(02)00159-6.
Hamdaoui O, Chiha M. Removal of methylene blue from aqueous solutions by wheat bran. Acta Chim Slov. 2007;54(1):407-18.
Husseien M, Amer AA, El-Maghraby A, Taha NA. Utilization of barley straw as a source of activated carbon for removal of methylene blue from aqueous solution. J Appl Sci Res. 2007;3(11):1352-8.
Aarfane A, Salhi A, Krati ME, Tahiri S, Monkade M, Lhadi EK. Etude cinetique et thermodynamique de l’adsorption des colorants Red195 et Bleu de methylene en milieu aqueux sur les cendres volantes et les machefers [Kinetic and thermodynamic study of the adsorption of Red195 and methylene blue dyes on fly ash and bottom ash in aqueous medium]. J Mater Environ Sci. 2014;5(6):1927-39.
Salhi A. Adsorption of methylene blue and Red195 dyes in aqueous medium by palm bark and sugarcane b Agasse: kinetic and thermodynamic study. J Mater Environ Sci. 2015;6(10):2944-57. doi: 10.13140/RG.2.1.3759.0481.
Cao JS, Lin JX, Fang F, Zhang MT, Hu ZR. A new absorbent by modifying walnut shell for the removal of anionic dye: kinetic and thermodynamic studies. Bioresour Technol. 2014;163(1):199-205. doi: 10.1016/j.biortech.2014.04.046, PMID 24813388.
Khelifi O, Mehrez I, Salah WB, Salah FB, Younsi M, Nacef M. Study of methylene blue (mb) adsorption from aqueous solutions on biosorbent prepared from Algerian datte stones. LARHYSS J. 2016;28(1):135-48.
Yadav S, Tyagi DK, Yadav OP. Equilibrium and kinetic studies on adsorption of aniline blue from aqueous solution onto rice husk carbon. Int J Chem Res. 2011;2(3):59-64.
Bhattacharyya K, Sharma A. Kinetics and thermodynamics of methylene blue adsorption on neem leaf powder. Dyes and Pigments. 2005;65(1):51-9. doi: 10.1016/j.dyepig.2004.06.016.
Srivastava R, Rupainwar DC. Eucalyptus bark powder as an effective adsorbent: evaluation of adsorptive characteristics for various dyes. Desalination and Water Treatment. 2009;11(1-3):302-13. doi: 10.5004/dwt.2009.864.
Afroze S, Sen TK, Ang M, Nishioka H. Adsorption of methylene blue dye from aqueous solution by novel biomass Eucalyptus Sheathiana Bark: equilibrium, kinetics, thermodynamics and mechanism. DWT. Desalination and Water Treatment. 2016;57(13):5858-78. doi: 10.1080/19443994.2015.1004115.
Dave P, Kaur S, Khosla E. Removal of eriochrome black-t by adsorption onto eucalyptus bark using green technology. Indian J Chem Technol. 2011;18(1):53-60.
Dawood S, Sen TK, Phan C. Synthesis and characterization of novel-activated carbon from waste biomass pine cone and its application in the removal of congo red dye from aqueous solution by adsorption. Water Air Soil Pollut. 2014;225(1):3-16. doi: 10.1007/s11270-013-1818-4.