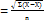Int J Chem Res, Vol 7, Issue 3, 5-10Research Article
SPECTROPHOTOMETRIC FINGERPRINTING AND CHEMICAL DETERMINATION OF STREPTOMYCIN, AMIKACIN, NEOMYCIN, AND GENTAMYCIN SULPHATE BY CONDENSING WITH NINHYDRIN REAGENT
EDEBI N. VAIKOSEN1, SAMUEL J. BUNU1,2*, EBIERE DODE1, RUTH B. EFIDI1
1Department of Pharmaceutical and Medicinal Chemistry, Faculty of Pharmacy, Niger Delta University, Wilberforce Island, Bayelsa State Nigeria. 2Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai-201203, China
Email: [email protected]
Received: 12 Apr 2023 Revised and Accepted: 28 May 2023
ABSTRACT
Objective: The study aimed to develop a simple, efficient, inexpensive, rapid, and reproducible spectrophotometric analytical technique for aminoglycosides analysis by condensing with ninhydrin reagent.
Methods: At a pH of 8.0, different aminoglycosides, including amikacin, gentamycin, neomycin, and streptomycin, were deaminated by the ninhydrin reagent. The working standard solution for each component was accurately placed into a series of 10 ml calibrated volumetric flasks, then 1 ml of the ninhydrin reagent was added and heated in a water bath for 15 min. The wavelength(s) of maximum absorption(s) was recorded after scanning the resultant purple complex from 350 nm to 930 nm. The final working concentrations ranged between 1600 and 2000 ug/ml.
Results: The average weight of a neomycin sulphate tablet was 0.747g, with a standard deviation of 0.667%. After complexing with ninhydrin reagent at pH 6.0 for 10 to 15 min, all aminoglycosides developed purple coloration that lasted beyond 24 h, compared to the initial white, pale yellow, and colorless appearance of streptomycin, amikacin, neomycin, and gentamycin, respectively. The scanned spectra of the purple complex of ninhydrin formed after reaction with aminoglycosides in the visible region (350-900 nm) were similar, indicating the presence of a common parent structural moiety. At 550 nm, 650 nm, 750 nm, and 850 nm, distinct absorptions were observed. Amikacin, Streptomycin, and Gentamicin had the highest absorbance between 800 and 900 nm. After the reaction with ninhydrin, three distinct absorbances were observed in the Neomycin spectrum: between 380 and 400 nm, 580 and 600 nm, and around 800 nm. The comparative spectra for the four aminoglycosides and ninhydrin reagent with blank show a unique feature for the compounds. The coefficients of regression were 0.996 and 0.995, respectively.
Conclusion: The proposed methods for analyzing streptomycin in streptomycin injections were successful. The percentage purity ranged from 017–110 % at 650 and 850 nm, which corresponds to the British Pharmacopoeia (BP) limit that streptomycin sulphate tablets should be between 97.00 and 110%.
Keywords: Aminoglycoside, Gentamycin, Amikacin, Neomycin, Streptomycin, Ninhydrin, λmax
© 2023 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/)
DOI: http://dx.doi.org/10.22159/ijcr.2023v7i3.221. Journal homepage: https://ijcr.info/index.php/journal
INTRODUCTION
Antibiotics are used to treat and prevent bacterial infections [1]. They are classified based on their chemical structural activity spectrum and mechanism of action [2]. Aminoglycosides inhibit bacterial protein synthesis. For some gram-negative aerobes and certain anaerobic bacilli, aminoglycosides are bactericidal [3, 4]. Different aminoglycosides are distinguished by the amino sugars linked to the aminocyclitol [5, 6].
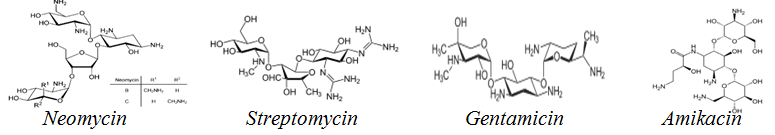
Fig. 1: Structures of some aminoglycoside antibiotics
Aminoglycoside antibiotics (fig. 1), are connected to amino sugar alterations at the 4, 5, and 4-positions via a non-sugar 2-deoxystreptamine scaffold. The two most significant families of aminoglycoside antibiotics are 4-and 6-disubstituted 2-deoxystreptamine derivatives [7, 8]. They are weakly absorbed through the gastrointestinal tract (GI) tract but are rapidly and completely absorbed intramuscularly. Peak serum aminoglycoside concentrations occur 30-120 min following intramuscular doses [9]. They are prevalent in renal tissue and have a protein binding fraction of 10%. The kidney typically excretes aminoglycosides unaltered via glomerular filtration. A little quantity of aminoglycoside is excreted via the bile. The serum half-life in persons with normal renal function is around 2-3 h [5].
In the Ultra-Violet-Visible (UV/Vis) area of the electromagnetic spectrum, absorption in the visible range has a direct influence on the perceived kind of sample [10]. Chemical derivatization is a spectrophotometric analysis process that converts a chemical substance into a comparable chemical structure result [11]. The bulk of chemical derivatization processes involves the conversion of analytes to compounds with longer chromophores, resulting in apparent absorption [11]. Colorimetric methods based on the fast reaction between amines and carbonyl compounds under proper circumstances to form Schiff's bases, hydrazones, semicarbazones, or oximes are used in the condensation reaction, which is a derivatization process [12, 13].
Most Pharmacopeia’s recommend microbiological method assays for aminoglycoside analysis [14], and bio-assay determination [15]. Previous research found that gentamicin could be determined after modifying with an ophthal aldehyde reaction [16]. In the presence of methyl and propyl hydroxybenzoate, the method was found to have high specificity to gentamicin. [17]. Another method was based on the ammonium molybdate oxidation of amantadine hydrochloride, which was found to be superior to previously reported ion-pair formation-based methods and pre-derivatization procedures [18]. The condensation of aminoglycosides with ninhydrin has few reports; thus we were able to develop a simple, efficient, inexpensive, rapid, and reproducible spectrophotometric analytical technique for streptomycin analysis by condensing with ninhydrin reagent.
MATERIALS AND METHODS
Apparatus and reagents
JENWAY 6305 model ultraviolet-visible spectrophotometer, analytical weighing balance, and previously calibrated Micropipettes (JENCONS Scientific Ltd) were used. JHD of China produced the methanol and chloroform, while Sigma-Aldrich of Switzerland produced the acetonitrile. The British Drug House (BDH) in England produced the ammonia solution, and KEM LIGHT produced the ninhydrin (98.5%). Reagent solutions were made with distilled water. All of the chemicals used were of analytical quality. The method reported by Vaikosen et al., [19], with few modifications, was adopted in the study.
Preparation of samples for analysis
A 5%(v/v) ammonia solution was created in a 100 ml volumetric flask by adding 20 ml of concentrated ammonia solution to 80 ml of water. In addition, 0.25g of ninhydrin was dissolved in 30 ml of 2% (w/v) sodium carbonate solution and diluted to 50 ml to make a 0.02M ninhydrin reagent. Then, 0.106 g of sodium carbonate was dissolved in 30 ml of distilled water and diluted to 50 ml to make a 0.02M (2%w/v) sodium carbonate solution. The aminoglycoside brands were obtained from trustworthy pharmacies in Yenagoa and Amassoma, Bayelsa State (table 1).
Table 1: Different aminoglycoside antibiotics used in the analysis
| S. No. | Drug | Dosage form | Strength | Manufacturer | Batch No | NAFDAC No. | Expiry date |
| 1 | Amikacin sulphate | Injection ampoule | 500 mg/2 ml | Pharma Aid Waren Handels Gmbh Harmburg-Germany | n-5306 | pb/drugs/1804-b | 04/2018 |
| 2 | Gentamicin sulphate | Injection ampoule | 80 mg/2 ml | ga 0886 | 01/2021 | ||
| 3 | Neomycin sulphate | oral tablets | 500 mg/tablet | Nem laboratories | nt14-86 | a4-0630 | 10/2018 |
| 4 | Streptomycin sulphate | injection ampoule | 5g powder/vial | North china pharmaceuticals co Ltd Shijiazhuang, China | 170506 | b4-0728 | 05/2020 |
Neomycin sulphate tablets preparation
Three neomycin sulphate pills were weighed and ground with a clean mortar and pestle. 500 mg of powdered neomycin sulphate was dissolved in 20 ml of water in a volumetric flask with steady shaking for 15 min. The liquid was then filtered, and 50 ml of more water was added.
Amikacin sulphate and gentamycin sulphate ampoules preparation
30 ml of 5%v/v ammonia solution was used in a 250 ml separating funnel to manufacture alkaline 500 mg amikacin sulphate and 800 mg gentamicin sulphate ampoules. The alkaline solution was extracted using four (4) volumes of 10 ml chloroform. Filter paper and anhydrous NaSO4 were used to filter the chloroform extract. The chloroform extracts were all mixed. The filtrate was air-dried at room temperature, and the residue was desiccated. To obtain 20,000ug/ml working stock solutions, the dried extract was dissolved in 2 ml methanol, transported to 25 ml volumetric flasks, and brought up to volume using acetonitrile.
Streptomycin vial preparation
30 ml of 5%v/v ammonia solution was used in a 250 ml separating funnel to alkalinize 1 g (1.015 g) of streptomycin vial. The released aminoglycoside was extracted with four (4) volumes of 10 ml chloroform. The chloroform extracts were mixed. At room temperature, the filtrate was air-dried, and the residue was desiccated. To acquire a working stock concentration of 40,000ug/ml, the dried extract was diluted in 2 ml methanol and quantitatively transferred into a clean and dry 25 ml volumetric flask before being brought up to volume with acetonitrile.
Reaction with ninhydrin reagent for spectroscopic analysis
With a few modifications, the procedures described by Vaikosen et al., [19], were followed. After properly transferring an aliquot amount of each aminoglycoside's working standard solution into a series of 10 ml calibrated volumetric flasks, 1 ml of the ninhydrin reagent was added and cooked in a boiling hot water bath for 15 min. The pH of the complex was measured, and the volume was filled with acetonitrile. The purple complex that resulted was then scanned from 350 nm to 930 nm. The maximum absorption wavelength(s) were recorded. The final working concentrations ranged between 1600 and 2000ug/ml.
RESULTS
Weight uniformity of neomycin sulphate tablets
The weight uniformity for the neomycin sulphate tablet is as presented in table 2 below:
Table 2: Weight uniformity of neomycin sulphate tablets
| S. No. | Weight of each tablet X (g) | Weight variation (X-X)g | Σ(X-X)2g | Percentage variation 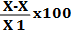 |
Standard Deviation (SD) |
Percentage deviation from the mean |
| 1 | 0.743 | -0.004 | -0.000016 | -0.538 | -0.000007g +0.0015 |
0.0015 x 100% 2.24 = 0.667% |
| 2 | 0.747 | 0 | 0 | 0 | ||
| 3 | 0.750 | 0.003 | 0.00009 | 0.4 | ||
| Total | 2.24 | -0.001 | 0.000007 | |||
| Mean (Σx) | 0.747 | -0.0003 | -0.0000023 | |||
| The average weight of Neomycin sulphate tablets | 2.24+0.0015g |
Key: x = mean, n = number of variables, and Σ = sum of Average weight of Neomycin sulphate tablets = 2.24g
The reaction of aminoglycosides with ninhydrin reagent
Physicochemical properties complex formation: Some physicochemical properties (before and after reactions of aminoglycosides with Ninhydrin) are presented in table 3.
Table 3: Physicochemical properties of aminoglycosides before and after reaction with Ninhydrin
| Parameters | Streptomycin sulphate | Amikacin | Gentamycin | Neomycin |
| Dosage form | Powder/solid | Liquid | Liquid | Tablet (solid) |
| Color of the drug before the reaction | White | Pale yellow | Colourless | Pale yellow |
| Color after complexing with 1 ml ninhydrin reagent | Purple | Purple | Purple | Purple |
| the pH of the complex formed | 6 | 6 | 6 | 6 |
| Time taken for color formation | 10-15 min | 10-15 min | 10-15 min | 10-15 min |
| Duration of color | >24 h | >24 h | >24 h | >24 h |
Light absorption wavelength determination of the complex for aminoglycosides
Spectral obtained for the four (4) aminoglycosides reacted with 10 ninhydrin reagents. Distinct absorptions were observed at 550 nm, 650 nm, 750 nm, and 850 nm
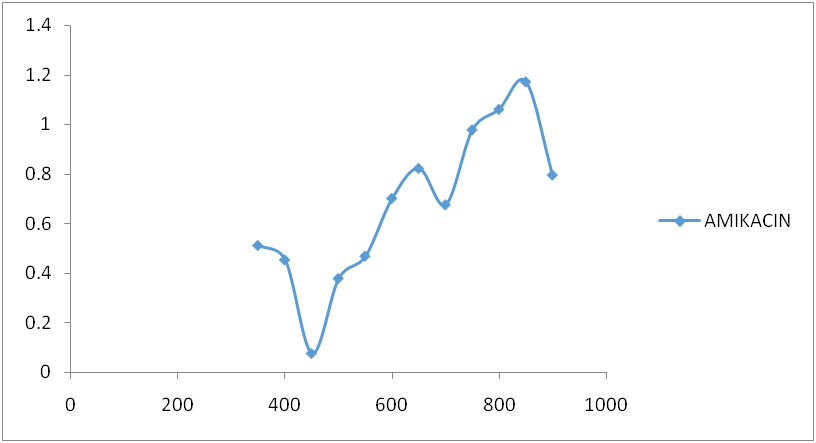
Fig. 3: Amikacin spectrum after reaction with ninhydrin
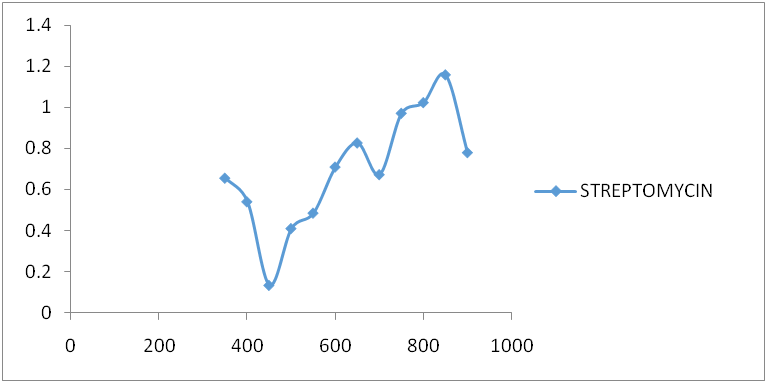
Fig. 4: Streptomycin spectrum after reaction with ninhydrin
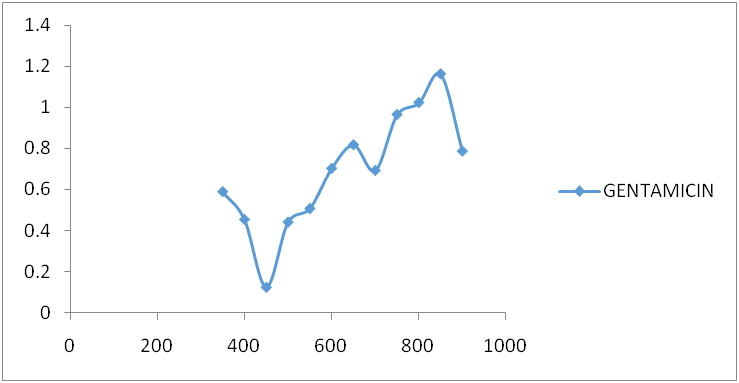
Fig. 5: Gentamicin spectrum after reaction with ninhydrin
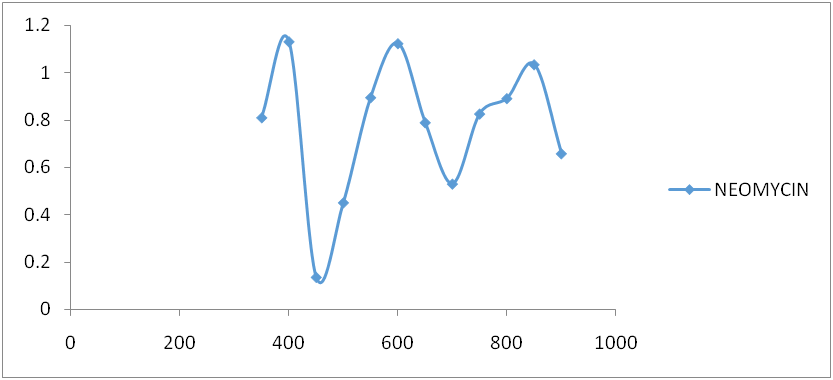
Fig. 6: Neomycin spectrum after reaction with ninhydrin
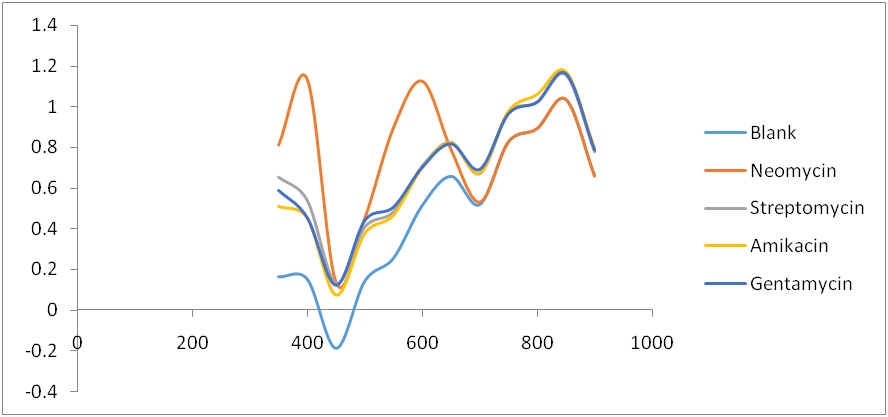
Fig. 7: Comparative spectra for four Aminoglycosides and Ninhydrin reagent blank
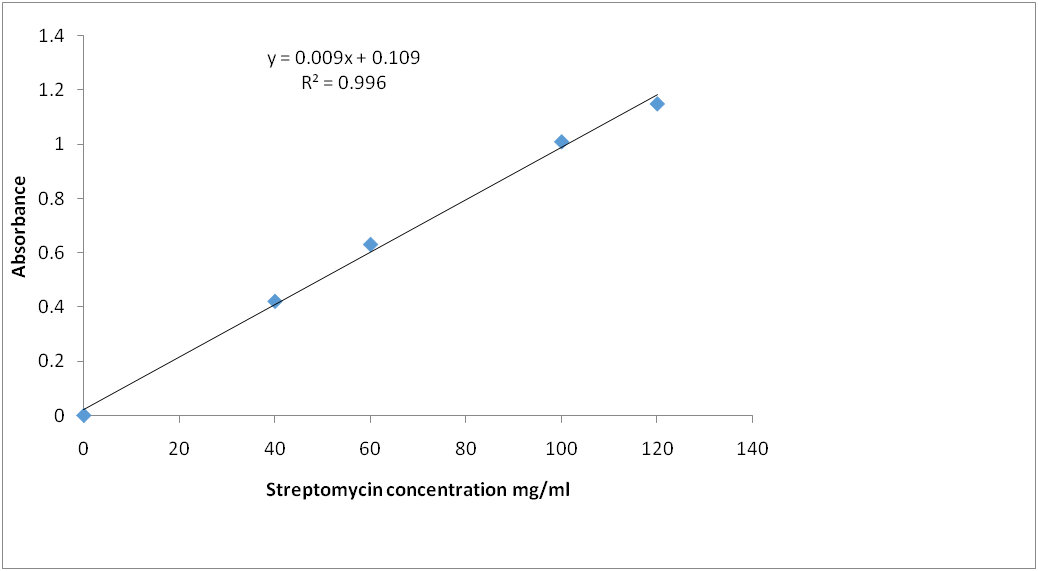
Fig. 8: Streptomycin calibration curve for reaction with ninhydrin (λ=850 nm)
Calibration curve for streptomycin standard
Calibration graph for curve for streptomycin standard after reaction with ninhydrin reagent: The standard calibration curves for streptomycin over a working concentration range 0ug/ml-120ug/ml at wavelengths (λ) 650 and 850 nm are presented in fig. 8 and 9, respectively.
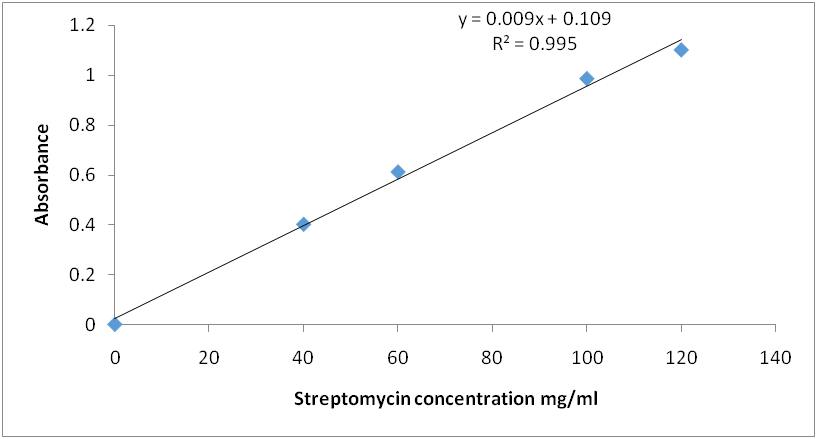
Fig. 9: Calibration curve for streptomycin reaction with ninhydrin reagent (λ=650 nm)
Methods validation and determination of the most appropriate wavelength for measurement
Using the equation of the straight line: A = bc+a; A = absorbance, b = slope, c = concentration, a = intercept.
Determination of percentage purity
y = 0.009x+0.023; x = actual concentration and y = absorbance
Average absorbance for Strepa® by North China pharmaceuticals = 1.018
![]()
% purity = Observed weight × 100/Expected weight
Expected weight = 100 mg; % purity = 101%
Table 4: Qualitative parameter and statistical data for the spectrophotometric determination of streptomycin by the proposed methods
| Parameters | Wavelength | |
| 650 nm | 850 nm | |
| Linear range/ug/ml | 400-1200 | 400-1200 |
| Intercept | 0.000 | 0.000 |
| Regression coefficient | 0.995 | 0.996 |
DISCUSSION
The average weight of a neomycin sulphate tablet was 0.747g, with a standard deviation of 0.667%. After complexing with ninhydrin reagent at pH 6.0 for 10 to 15 min, all aminoglycosides developed a purple coloration that lasted for more than 24 h, compared to the initial white, pale yellow, and colorless appearance of streptomycin, amikacin, neomycin, and gentamycin, respectively. After reacting the four (4) aminoglycosides with ten ninhydrin reagents, a spectral line was obtained.
Amikacin, gentamycin, neomycin, and streptomycin were all deaminated by ninhydrin reagent in an alkaline medium (pH 6.0) to form a purple product. The scanned spectra of the purple complex of ninhydrin formed after reaction with aminoglycosides in the visible region (350-900 nm) were similar, indicating the presence of a common parent structural moiety. At 550 nm, 650 nm, 750 nm, and 850 nm, distinct absorptions were observed. Amikacin, Streptomycin, and Gentamicin had the highest absorbance between 800 and 900 nm (fig. 3, fig. 4, and fig. 5). After the reaction with ninhydrin, three distinct absorbances were observed in the Neomycin spectrum: between 380 and 400 nm, 580 and 600 nm, and around 800 nm (fig. 6). The comparative spectra for the four aminoglycosides and ninhydrin reagent with blank show a unique feature for the compounds (fig. 7). The standard calibration curves for streptomycin over a working concentration range 0ug/ml-120ug/ml at wavelengths (λ) 650 and 850. The streptomycin calibration curve for the ninhydrin reaction at 850 nm yielded a regression of 0.996 (fig. 8), while 0.996 was obtained at 650 nm (fig. 9). The straight-line equation was used to validate and determine the best wavelength for measuring aminoglycosides, whereas the regression equation was used to calculate the percentage purity and composition of streptomycin under assay. As expected, the qualitative parameter and statistical data for the suggested techniques of spectrophotometric measurement of streptomycin demonstrated zero interception at both wavelengths (650 nm and 850 nm), and a linear range of 400-12000 at both wavelengths.
All aminoglycosides include both primary and secondary amino sugars. All aminoglycosides displayed four unique peaks of light absorbance after the ninhydrin reaction at 550, 650, 750, and 850 nm. However, at 650 and 850 nm, the two were most distinguishable, with the latter displaying the most significant absorbance (λmax). When compared to the aminoglycosides, the absorbance values obtained for the ninhydrin blank were generally lower. The spectral for the ninhydrin reagent concerning streptomycin was discovered to be concentration dependent. This observation is consistent with previous reports [6]. Beer's Law states that absorbance is proportional to the concentration of the sample being analyzed [20]. The differential in concentration may have a function of differences in the number of–NH2 and–OH groups attached to the hexose rings. This is relative to precious reports on flavonoids, which exhibited two distinct bands in the UV/vis region, where the first band was attributed to the cinnamoyl moiety while the second band was the benzoyl moiety [21]. Thus, the results obtained in the spectrum above contain a single unique concentration of the compounds analyzed.
Before using the method to analyze streptomycin injections, the method's suitability, medium, volumes, concentrations, and order of addition of the reagents were determined. The proposed method was used to test the dosage form of streptomycin injections. Streptomycin was used as a case study, and an increase in streptomycin concentration resulted in a linear increase in absorbance. The coefficients of regression were 0.996 and 0.995, respectively. The obtained results were compared between the two wavelengths.
CONCLUSION
Although the aminoglycosides were removed from their dosage forms at first, the presence of excipients did not affect the formation of blue-colored complexes or the linearity of the calibration graphs. The proposed methods for analyzing streptomycin in streptomycin injections were successful. At 650 and 850 nm, the percentage purity ranged. Streptomycin sulphate tablets should be within the range of 017-110, which corresponded to the USP29-NF-24 limit of 97.00-110%. Thus, the procedure is simple, accurate, quick, and inexpensive, as well as repeatable.
CONFLICT OF INTERESTS
The authors hereby declare no conflict of interests.
AUTHORS CONTRIBUTIONS
All authors participated in research, manuscript writing and approval processes.
REFERENCES
-
Jian W, James D, Jack MN. Chemical analysis of antibiotics in food. 1st ed. John Wiley & Sons, Inc; 2012. p. 1-60.
-
Finberg RW, Moellering RC, Tally FP, Craig WA, Pankey GA, Dellinger EP. The importance of bactericidal drugs: future directions in infectious disease. Clin Infect Dis. 2004;39(9):1314-20. doi: 10.1086/425009. PMID 15494908.
-
Munita JM, Arias CA. Mechanisms of antibiotic resistance. Microbiol Spectr. 2016;4(2). doi: 10.1128/microbiolspec.VMBF-0016-2015. PMID 27227291.
-
Bharadwaj A, Rastogi A, Pandey S, Gupta S, Sohal JS. Multidrug-resistant bacteria: their mechanism of action and prophylaxis. BioMed Res Int. 2022;2022:5419874. doi: 10.1155/2022/5419874, PMID 36105930.
-
Mingeot Leclercq MP, Glupczynski Y, Tulkens PM. Aminoglycosides: activity and resistance. Antimicrob Agents Chemother. 1999;43(4):727-37. doi: 10.1128/AAC.43.4.727, PMID 10103173.
-
Krause KM, Serio AW, Kane TR, Connolly LE. Aminoglycosides: an overview. Cold Spring Harb Perspect Med. 2016;6(6):a027029. doi: 10.1101/cshperspect.a027029. PMID 27252397.
-
Durante Mangoni E, Grammatikos A, Utili R, Falagas ME. Do we still need the aminoglycosides? Int J Antimicrob Agents. 2009;33(3):201-5. doi: 10.1016/j.ijantimicag.2008.09.001, PMID 18976888.
-
Garneau Tsodikova S, Labby KJ. Mechanisms of resistance to aminoglycoside antibiotics: overview and perspectives. Medchemcomm. 2016;7(1):11-27. doi: 10.1039/C5MD00344J, PMID 26877861.
-
Dager WE. Aminoglycoside pharmacokinetics: volume of distribution in specific adult patient subgroups. Ann Pharmacother. 1994;28(7-8):944-51. doi: 10.1177/106002809402800719, PMID 7949517.
-
Skoog DA, Holler F. Belmont, CA. 6th ed. Crouch: James Publishing, Stanley R. Principles of Instrumental Analysis. Thomson; 2007. p. 169-73.
-
Rocha FRP, Zagatto EAG. Chemical derivatization in flow analysis. Molecules. 2022;27(5):1563. doi: 10.3390/molecules27051563, PMID 35268664.
-
Bagade SB. Sanjay, K.P. spectrophotometric estimation of torsemide in tablet dosage form using chemical derivatization technique [research article]. J Pharm OA. 2010;2(1):52-5.
-
Rowe WF. Forensic chemistry. Kirk Othmer Encycl Chem Technol. 2015;1-19:3. doi: 10.1002/0471238961.0615180506091908.a01.
-
British Pharmacopoeia. Vol. II. Her Majesty's Stationery Office, London; 1998. p. XIV A205-A210.
-
Hanes SD, Herring VL. Gentamicin enzyme-linked immunosorbent assay for microdialysis samples. Ther Drug Monit. 2001;23(6):689-93. doi: 10.1097/00007691-200112000-00016, PMID 11802105.
-
Stypulkowska K, Blazewicz A, Fijalek Z, Sarna K. Determination of gentamicin sulphate composition and related substances in pharmaceutical preparations by LC with charged aerosol detection. Chromatographia. 2010;72(11-12):1225-9. doi: 10.1365/s10337-010-1763-y, PMID 21212825.
-
Krzek J, Woltynska H, Hubicka U. Determination of gentamicin sulphate in injection solutions by derivative spectrophotometry. Analytical Letters. 2009;42(3):473-82. doi: 10.1080/00032710802424461.
-
Darwish IA, Khedr AS, Askal HF, Mahmoud RM. Simple and sensitive spectrophotometric methods for determination of amantadine hydrochloride. J Applied Spec. 2006;73(6):707-12.
-
Vaikosen EN, Origbo SO, Ere D, Odaderia P. Comparative application of biological and ninhydrin- derivatized spectrophotometric assays in the evaluation and validation of amikacin sulfate injection. Braz J Pharm Sci. 2022;58:e201185, doi: 10.1590/s2175-97902022e201185.
-
Beer. Bestimmung der absorption des rothen lichts in farbigen flussigkeiten [Determination of the absorption of red light in colored liquids]. Annalen Phys. 1852;162(5):78-88. doi: 10.1002/andp.18521620505.
-
Ibibia ET, Bankole TA, Ogunjimi DV, Mmuo AI, Amuda MO. GC-MS profile, synthesis and spectral characterization of flavonoid-metal (cadmium, cobalt, copper, nickel) complexes of jatropha Curcus leaves. Int J Chem Res. 2023;7(2):4-12. doi: 10.22159/ijcr.2023v7i2.217.