
Int J Chem Res, Vol 6, Issue 2, 1-5Research Article
SYNTHESIS AND SPECTROSCOPIC CHARACTERIZATION OF SILVER (I) MEBENDAZOLE COMPLEXES
OLUFUNSO O. ABOSEDE, LOVETH E. EZEGWU
Department of Chemistry, Federal University Otuoke, PMB 126, Yenagoa, Bayelsa State, Nigeria
Email: [email protected]
ABSTRACT
Objective: The aim of this research work is to synthesize silver-mebendazole complexes with imidazole (imi), 2,2-bipyridine (bpy) and 1,10-phenanthroline (phen) as secondary ligands that will be active and effective against microbes.
Methods: Environmentally friendly techniques were employed to synthesize the new complexes by reacting silver nitrate and ligands in water and at ambient conditions. The formed complexes were isolated and dried at ambient conditions and characterized by UV and FTIR spectroscopies; the physicochemical and antimicrobial properties of the complexes against Proteus mirabilis and Salmonella typhi were also tested by agar well diffusion method.
Results: Spectroscopic studies confirmed the formation of the complexes. In the newly synthesized complexes, silver is coordinated by N of the imidazole moiety of mebendazole as well as N atoms of imidazole, 2,2-bipyridine and 1,10-phenanthroline. The complexes are stable and possess low aqueous solubility. Antimicrobial analyses revealed that they were active against the tested organisms with antibacterial activities higher than the parent drug, mebendazole.
Conclusion: The newly synthesized complexes were stable and the results show their antimicrobial potential. The resultant antimicrobial activity of the complexes is as a result of synergistic activities of silver and the ligands.
Keywords: Synthesis and spectroscopic, Silver (I) mebendazole complexes
© 2022 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open-access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/)
DOI: http://dx.doi.org/10.22159/ijcr.2022v6i2.197. Journal homepage: https://ijcr.info/index.php/journal
INTRODUCTION
Mebendazole is a stable, water-insoluble, over-the-counter, inexpensive drug that has been in use since 1970s as a broad-spectrum anthelmintic for the treatment of a range of parasitic infections, including threadworm, whipworms, tapeworms, hookworms, roundworms, ascaris and other nematodes and trematodes such as trichuriasis, strongyloidiasis, and enterobiasis [1]. In addition, mebendazole’s anti-tumor properties have been established in vitro and in animal models against a variety of cancer cell lines such as liver, lung, ovary, prostate, colorectal, breast, head and neck cancers, and melanoma [2]; its effectiveness in overcoming cisplatin resistance in human ovarian cancer cells has also been demonstrated [3]. Moreover, a number of clinical trials of mebendazole as an anticancer drug, either alone or in combination with standard chemotherapy drugs, are currently ongoing [4].
The increasing epidemic in different regions of the world, including the current COVID-19 pandemic has amplified the demand and continuous search for potent drugs and alternative therapies for combatting diseases that affect the populace and the menace of drug resistance by microbes. Conversely, there is the decline in antimicrobial drug development in recent times because of the issue of antimicrobial resistance and cost of developing of new drugs. This has led scientists to resort to other options including the development of metal-based drugs [5].
One of the schemes to meeting this growing demand for pharmaceuticals is metal-containing therapeutics. Transition metal complexes are essential in medicine and biology. Examples include haemoglobin, an iron complex that transports oxygen in the blood, and complexes of Fe, Zn, Cu and Mo, which are crucial components of certain enzymes and serve as catalyst for most biological reactions. Notable among the uses of metal complexes in biology and medicine is their use as therapeutic and diagnostic agents. Metal-based drug represents a novel group of antimicrobial agents with potential application for the control of bacterial, fungal and parasitic infections.
Silver and silver compounds have long been generally reputable for their antimicrobial properties for centuries ago [6, 7] and a number of silver compounds are in clinical use while some are currently in clinical trials [8-11]. Barnet Rosenberg’s discovery of the anticancer activity of cisplatin in 1965 and other metal-containing derivatives being developed and used clinically have reinvigorated the development of metallopharmaceuticals for therapeutic uses suc as antibacterial, anti-inflammatory and antiseptic, while some of these compounds have been found capable of combating drug-resistant bacteria, fungi and parasites [12]. Among the class of metallopharmaceuticals, silver complexes and silver-containing compounds occupy a special place because of their relative ease of preparation, characterization, stability and low toxicity.
Silver complexes with a number of different ligands such as N-heterocyclic carbenes, phosphines, imidazole, pyridines, etc. have been extensively studied for their excellent antibacterial properties and have been shown to be more effective and less toxic than some organic-based drugs [5]. Antibacterial experiments have shown broader antimicrobial activity for silver complexes with Ag-O and Ag-N bonds [13, 14] and the propensity of these complexes as antitumor agents have been established in vitro. Based on the type of ligand coordinated to silver (I), Ag(I) complexes have been shown to possess selective cytotoxicity and antimicrobial activity towards different cells and organisms [15]. In view of the antimicrobial potentials of metal complexes [16, 17], silver (I) complexes of mebendazole have been synthesized with the aim of obtaining new complexes of therapeutic value against selected organisms.
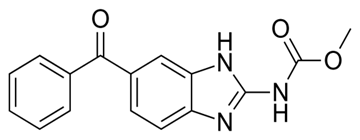
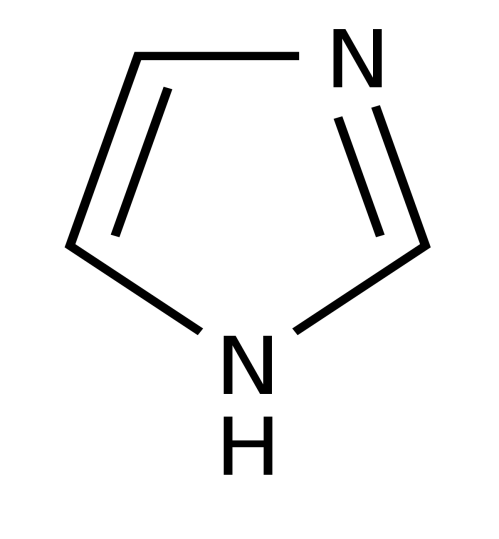
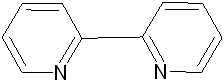
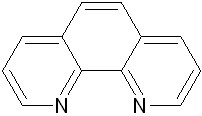
Mebendazole Imidazole 2,2’-bipyridine 1,10-phenanthroline
Scheme 1: Structures of ligands used
MATERIALS AND METHODS
All reagents (mebendazole, 1, 10-phenanthroline, 2,2’-bipyridine and imidazole), solvents (methanol ethanol, dimethylsulphoxide) and metal salt (AgNO3) used in this research were of analytical reagent grade and were procured from VWR International and used without further purifications. UV-Visible spectra were recorded on a JascoV-730 UV-Visible spectrophotometer and infrared spectra were recorded in a range 4000-400 cm-1 on Shimadzu FTIR-8400S on samples pressed in KBr pellet at Redeemer University of Nigeria, Osun State. Magnetic stirring was carried out using the Tecno-Vetro 20052 Monza magnetic stirrer. Reactions involving silver nitrate were conducted away from light.
Synthesis of complexes
[AgMeb]NO3 (1): 10 ml of 0.2 mol NaOH was added to 0.266 g (1 mmol) of mebendazole in a round bottom flask and stirred at room temperature for 20 min. On addition of 0.18 g (1 mmol) of AgNO3, the colour changed to brown. Aluminum foil was used to cover the reaction vessel and the reaction mixture was stirred for 30 min by which time the colour changed to green. The solution was stirred for a further 10 h without any further changes and the resulting product was filtered and kept in the dark. The residue was allowed to dry for 5 d at room temperature. Yield (residue): 0.38 g, 81%. UV-Vis (CH3OH): 225.5 nm, (DMSO): 272 nm.
[AgMebimi]NO3 (2): 10 ml of 0.2 mol NaOH was added to 0.266 g (1 mmol) of mebendazole and stirred at room temperature for 20 min. 0.18 g (1 mmol) of AgNO3 was introduced into the solution, which turned it brown. After 3 h of stirring, 0.068 g (1 mmol) of imidazole dissolved in 4.5 ml of water was added and the solution turned milky. The solution was stirred for more than 10 h and the resulting precipitate was filtered and kept in a cupboard; the residue was dried at room temperature for 5 d. Yield (residue): 0.339, 66%. UV-Vis (CH3OH): 221 nm, UV-Vis (DMSO): 273 nm.
[AgMebbpy]NO3 (3): 10 ml of 0.2 mol NaOH was added to 0.266 g (1 mmol) of mebendazole and stirred for 20 min at room temperature. 0.18 g (1 mmol) of AgNO3 was introduced into the mixture; then, the solution turned brown. 0.156 g (1 mmol) of 2, 2’-bipyridine was later added and the solution was stirred for 10 h without any further changes. The resulting precipitate was filtered and kept in the dark for 5 d at room temperature to dry. Yield: 0.39 g, 64%, UV-Vis (DMSO): 271.5 nm.
[AgMebphen]NO3: (4) 10 ml of 0.2 mol NaOH was added to 0.266 g (1 mmol) of mebendazole and stirred at room temperature. 0.18 g (1 mmol) of AgNO3 was introduced into the mixture. On addition of 0.198 g (1 mmol) of 1,10-Phenanthroline, 6 ml of water and 8 ml of the solution became light green. The mixture was stirred for 10 h without any further changes. The solution was filtered and kept in the dark. The residue was dried in the dark for 5 d at room temperature. Yield: 0.771g, 87%. UV-Vis (DMSO): 269.5 nm.
Antimicrobial studies
The complexes were tested for their antimicrobial activity by agar well diffusion method [18]. The pre-sterilized petri-dish containing 20 ml of nutrient agar was allowed to solidify. Then bacteria were introduced aseptically using striking plate method. A sterile cork borer was used to make wells in each of the plates. Suitable dilutions of the complexes were made with distilled sterile water and carefully placed in each well by micropipette. The plates were incubated at 37 °C for 24 h. Antimicrobial activity was evaluated by measuring the inhibition zones. Inhibition zones were recorded as the diameter of no growth area.
RESULTS AND DISCUSSION
The newly synthesized complexes 1-4 are not coloured as expected for silver (I) complexes and are stable at ambient conditions. Table 1 presents the solubility of the complexes and the parent ligand.
Table 1: Solubility of the ligands and metal complexes in different solvents
| Compounds | Water | Ethanol | Methanol | DMSO |
| Mebendazole | Insoluble | Slightly soluble | Insoluble | Soluble |
| Bipyridine | slightly soluble | Soluble | Soluble | Soluble |
| Phenanthroline | Soluble | Soluble | Soluble | Soluble |
| Imidazole | Soluble | Soluble | slightly soluble | Soluble |
| [Agmeb]NO3 (1) | Insoluble | Slightly soluble | Slightly soluble | Soluble |
| [AgmebImi]NO3 (2) | Insoluble | Slightly soluble | Slightly soluble | Soluble |
| [Agmebbpy]NO3(3) | Insoluble | Insoluble | Insoluble | Slightly soluble |
| [Agmebphen]NO3(4) | Insoluble | Insoluble | Insoluble | Slightly soluble |
The solubility result shows that mebendazole is insoluble in water but slightly soluble in ethanol, methanol and soluble in dimethylsulfoxide. Bipyridine, phenanthroline, and imidazole are soluble in DMSO, ethanol, methanol. The solubility of the metal complexes showed a different pattern whereby the solubility varied from insoluble in most solvents to slightly soluble in dimethylsulfoxide.
Electronic spectra
The complexes were characterized using UV-Vis spectroscopy by scanning from 200 nm to 600 nm since silver complexes will not show d-d bands between 400-800 nm. The electronic absorption spectral data of the metal complexes in dimethylsulfoxide are compiled in table 2.
Table 2: Physico-chemical parameters of the silver complexes
| Complex | Product (Yield %) | Colour | Appearance | Ligands transition (nm) |
| 1 | 87 | Off-white | amorphous | 272 |
| 2 | 66 | Off-white | amorphous | 221 |
| 3 | 87 | Off-white | amorphous | 269.5 |
| 4 | 64 | Off-white | amorphous | 271.5 |
FTIR spectra
The FTIR spectra of the newly synthesized metal complexes were taken on Shimadzu FTIR-8400S FTIR spectrometer in the region 400-4000 cm-1 and compared to the previously interpreted FTIR spectra of mebendazole [19-20]. The comparison of FTIR spectrum of each of the ligands with that of its respective metal complexes revealed certain characteristic differences. One of the significant diagnostic features to be expected between the IR spectra of the parent ligand and the respective metal complexes is the presence/absence of bands due to heteroatoms (oxygen and nitrogen of both the O-H and N-H groups of the ligands) which could be involved in coordination bond with the transition metal ion. The FTIR spectra of the free ligands mebendazole and its complexes are presented in the table below.
Table 3: Characteristic absorption bands of mebendazole and its complexes
| Mebendazole | [Agmeb]NO3 (1) | [AgmebImi]NO3 (2) | [Agmebpy]NO3 (3) | [Agmebphen]NO3 (4) | Assignment |
| 3369strong | 3369 | 3414, 3369 | 3369 | 3369 | N-H Aryl |
| 3059.20weak | 3061 | 3057 | 3055.35 | 3057.27 | C-H Aryl |
| 1732strong | 1732 | 1732 | 1732 | 17321 | C=O carbamate |
| 1637strong | 1637 | 1637 | 1637 | 1637 | C=O benzoyl |
| 1527.67medium | 1595, 1541 | 1595, 1529 | 1529.60 | 1541.18 | N-H in plane bending |
| 1456.30 medium | 1446 | 1458 | 1456.30 | 1444.73 | C-C heterocyclic Ar |
| 1263 strong | 1384w 1278, 1228 | 1276, 1228 | 1263 | 1276 | C-H in plane bending |
| 1193, 1118, 1091.75 | 1193, 1116 | 1193, 1118, 1091 | 1093.67 | C-O | |
| 1012.66, 977 | 1028, 977, 825 | 1012, 977, 825 | 1012.66 | 1028.09 | C-H in plane |
| 769.62 | 769 | 769 | 758.0 | 769.62 | N-H |
| 705.97 | 707 | 705 | 705.97 | 727.19 | N-H |
| 542 | 542 | 542 | 495.72 | 418.57 | Ag-N |
| 437 | 441 | 443 | 441.7 | Ag-N |
The comparison of the FTIR spectrum of mebendazole and the spectra of complexes 1, 2, 3 and 4 are discussed below. It was observed that there were some changes in the FTIR spectra of free mebendazole and complexes 1, 2, 3 and 4. First, N-H (carbamate) stretching of mebendazole at 3369 cm-1 remains unchanged in all the complexes. This shows that the point of coordination of silver to mebendazole is not with N-carbamate atom. The C=O (carbamate) stretching of mebendazole at 1732 cm-1 which remains unchanged for complexes 3 and 4 has provided evidence that this point is not the coordination. C=O (benzoyl) stretching, which appears at 1637 cm-1 for mebendazole also remains unchanged for the complexes.
These spectra features confirm that the coordination of silver to mebendazole is not with the carbamate and benzoyl moieties of mebendazole. However, there are significant changes in the spectra of the complexes at the imidazole moiety which indicates coordination of silver to mebendazole in this segment through the imidazole N atom. The spectrum of mebendazole shows a band at 1456 cm-1 corresponding to C-C heterocyclic aryl and this band shifted to 1446, 1458 and 1444 cm-1 for complexes 1, 2 and 4, respectively. Furthermore, for C-H (Ar) in-plane bending vibration, a strong band at 1263 cm-1 in FTIR spectrum of mebendazole shifted to higher wavenumbers in complexes 1, 2 and 4. C=N (aryl) for bpy and phen ligands in complexes 3 and 4 appears as sharp bands at 1323 cm-1 and 1384 cm-1 respectively.
New absorption bands of Ag-N appeared at 418.57 cm-1 and 441.71 cm-1 for the complexes, respectively showing there is silver to ligand bond formation via the N imidazole atom of mebendazole.
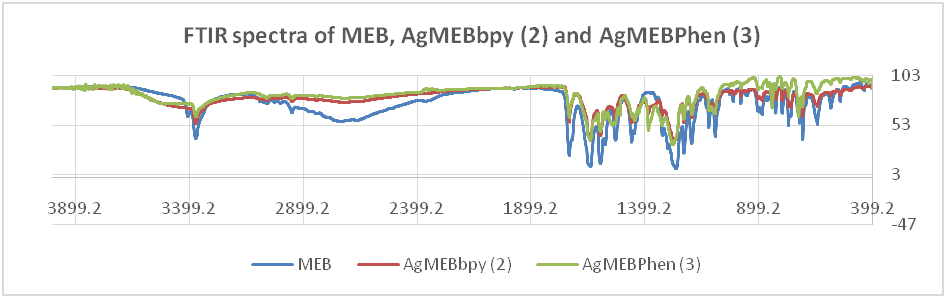
Fig. 1: FTIR spectra mebendazole and complexes 2 and 3
Antimicrobial activities
Table 4: Antimicrobial activity of mebendazole and the new complexes
| Antibiotics used 25 mg/ml | Bacterial species | Zone of inhibition (mm) | Result |
| Mebendazole | Proteus mirabilis Salmonella typhi |
22 - |
Highly sensitive |
| Ag-Meb (1) | Proteus mirabilis Salmonella typhi |
30 25 |
Highly sensitive Highly sensitive |
| Ag-Meb-Bpy (2) | Proteus mirabilis Salmonella typhi |
32 22 |
Highly sensitive Highly sensitive |
| Ag-Meb-Phen (3) | Proteus mirabilis Salmonella typhi |
30 20 |
Highly sensitive Highly sensitive |
| Ag-Meb-imi (4) | Proteus mirabilis Salmonella typhi |
30 25 |
Highly sensitive Highly sensitive |
Results are expressed as mean of triplicate sample.
Table 4 shows the activity of the new complexes against some bacteria. The antimicrobial analyses of the new complexes were tested for Proteus mirabilis and Salmonella typhi. Both organisms were discovered to be more sensitive to the new complexes than the parent drug. All the newly synthesized silver complexes demonstrated higher inhibitory activity against Proteus mirabilis than mebendazole, the parent ligand. Salmonella typhi is resistant to mebendazole but very sensitive to each of the newly synthesised complexes. This antibacterial role of the complexes is as a result of the synergy of antibacterial activities of the central metal and the secondary ligands.
CONCLUSION
Synthesis and characterisation of four complexes of silver with mebendazole as a primary ligand as well as silver with mebendazole and imidazole, 2.2’-bipyridine and 1, 10-phenanthroline have been achieved. The complexes were synthesized using environmentally friendly solvent and at ambient conditions. The resulting complexes were bound by N of the imidazole moiety of mebendazole to the silver ion. The new metal complexes exhibited significant inhibition against all tested microorganisms (Salmonella typhi and Proteus mirabilis); higher inhibition than the respective parent ligands was obtained for each of the complexes.
ACKNOWLEDGMENT
UV-Visible spectra were recorded on JASCO UV-Visible V730 spectrophotometer donated through IFS individual grant (IFS 5780-1) awarded to Olufunso O. Abosede by the International Foundation for Science (IFS), Stockholm, Sweden and the Organization for the Prohibition of Chemical Weapons (OPCW).
FUNDING
Nil
AUTHORS CONTRIBUTIONS
Both authors contributed equally to this work and approved the final manuscript.
CONFLICT OF INTERESTS
The authors have declared that no competing interests exist.
REFERENCES
Brugmans JP, Thienpont DC, van Wijngaarden I, Vanparijs OF, Schuermans VL, Lauwers HL. Mebendazole in enterobiasis. Radiochemical and pilot clinical study in 1,278 subjects. JAMA. 1971;217(3):313-6. doi: 10.1001/jama.1971.03190030039008, PMID 5109194.
Chai JY, Jung BK, Hong SJ. Albendazole and mebendazole as anti-parasitic and anti-cancer agents: an update. Korean J Parasitol. 2021;59(3):189-225. doi: 10.3347/kjp.2021.59.3.189, PMID 34218593.
Huang L, Zhao L, Zhang J, He F, Wang H, Liu Q, Shi D, Ni N, Wagstaff W, Chen C, Reid RR, Haydon RC, Luu HH, Shen L, He TC, Tang L. Antiparasitic mebendazole (MBZ) effectively overcomes cisplatin resistance in human ovarian cancer cells by inhibiting multiple cancer-associated signaling pathways. Aging (Albany, NY). 2021;13(13):17407-27. doi: 10.18632/aging.203232, PMID 34232919.
Son DS, Lee ES, Adunyah SE. The antitumor potentials of benzimidazole anthelmintics as repurposing drugs. Immune Netw. 2020;20(4):e29. doi: 10.4110/in.2020.20.e29. PMID 32895616.
Frei A, Zuegg J, Elliott AG, Baker M, Braese S, Brown C, Chen F, G Dowson C, Dujardin G, Jung N, King AP, Mansour AM, Massi M, Moat J, Mohamed HA, Renfrew AK, Rutledge PJ, Sadler PJ, Todd MH, Willans CE, Wilson JJ, Cooper MA, Blaskovich MAT. Metal complexes as a promising source for new antibiotics. Chem Sci. 2020;11(10):2627-39. doi: 10.1039/c9sc06460e, PMID 32206266.
Medici S, Peana M, Crisponi G, Nurchi VM, Lachowicz JI, Remelli M, Zoroddu MA. Silver coordination compounds: a new horizon in medicine. Coord Chem Rev. 2016;327-328:349-59. doi: 10.1016/j.ccr.2016.05.015.
Dai T, Huang YY, Sharma SK, Hashmi JT, Kurup DB, Hamblin MR. Topical antimicrobials for burn wound infections. Recent Pat Antiinfect Drug Discov. 2010;5(2):124-51. doi: 10.2174/157489110791233522, PMID 20429870.
Waszczykowska A, Zyro D, Ochocki J, Jurowski P. Clinical application and efficacy of the silver drug in ophthalmology: A literature review and new formulation of EYE drops with drug silver (I) complex of metronidazole with the improved dosage form. Biomedicines. 2021;9(2):210. doi: 10.3390/biomedicines9020210, PMID 33669740.
Trieu A, Mohamed A, Lynch E. Silver diamine fluoride versus sodium fluoride for arresting dentine caries in children: a systematic review and meta-analysis. Sci Rep. 2019;9(1):2115. doi: 10.1038/s41598-019-38569-9. PMID 30765785.
Peng JJ, Botelho MG, Matinlinna JP. Silver compounds used in dentistry for caries management: a review. J Dent. 2012;40(7):531-41. doi: 10.1016/j.jdent.2012.03.009. PMID 22484380.
Lansdown ABG. A pharmacological and toxicological profile of silver as an antimicrobial agent in medical devices. Adv Pharmacol Sci. 2010. p. 16. doi: 10.1155/2010/910686, PMID 21188244.
Khatoon N, Alam H, Khan A, Raza K, Sardar M. Ampicillin silver nanoformulations against multidrug-resistant bacteria. Sci Rep. 2019;9(1):6848. doi: 10.1038/s41598-019-43309-0, PMID 31048721.
Rusu A, Hancu G, Cristina Munteanu A, Uivarosi V. Development perspectives of silver complexes with antibacterial quinolones: successful or not? J Organomet Chem. 2017;839:19-30. doi: 10.1016/j.jorganchem.2017.02.023.
Liang X, Luan S, Yin Z, He M, He C, Yin L, Zou Y, Yuan Z, Li L, Song X, Lv C, Zhang W. Recent advances in the medical use of silver complex. Eur J Med Chem. 2018;157:62-80. doi: 10.1016/j.ejmech.2018.07.057, PMID 30075403.
Kuzderova G, Rendosova M, Gyepes R, Sovova S, Sabolova D, Vilkova M, Olejnikova P, Bacova I, Stokic S, Kello M, Vargova Z. Antimicrobial and anticancer application of silver (I) dipeptide complexes. Molecules. 2021;26(21):6335. doi: 10.3390/molecules26216335, PMID 34770744.
Vashi RT, Shelat CD. Synthesis, characterization and antifungal activity of 2[4(2,3-dichlorophenyl) piperazine1-yl methyl 3[8hydroxide quinoline5yl]3 (h)quinazolin4one ligand and its metal chelates. Int J Chem Res. 2010 Jan 1;1(2):11-4.
Heidari A. Investigation of cancer cells using thin layers of cadmium oxide (cdo)–dna/rna sandwiched complex composite plasmonic nanostructure under synchrotron radiation. Int J Chem Res. 2022 Mar 25;6(1):1-14. doi: 10.22159/ijcr.2022v6i1.180.
Direm A, Abdelbaky MSM, Sayın K, Cornia A, Abosede O, Garcia Granda S. Sev and pcu topological nets in one-pot newly synthesized mixed-ligand imidazole-containing Cu(II) coordination frameworks: crystal structure, intermolecular interactions, theoretical calculations, magnetic behavior and biological activity. Inorg Chim Acta. 2018;478:59-70. doi: 10.1016/j.ica.2018.03.011.
Chen J, Wang Z, Wu C, Li S, Lu T. Crystal engineering approach to improve the solubility of mebendazole. Cryst Eng Comm. 2012;14(19):6221-9. doi: 10.1039/c2ce25724f.
Calvo NL, Kaufman TS, Maggio RM. Mebendazole crystal forms in tablet formulations. An ATR-FTIR/chemometrics approach to polymorph assignment. J Pharm Biomed Anal. 2016 Apr 15;122:157-65. doi: 10.1016/j.jpba.2016.01.035. PMID 26874854.